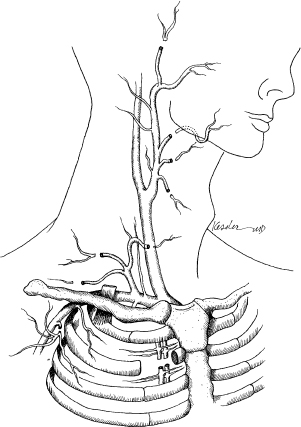
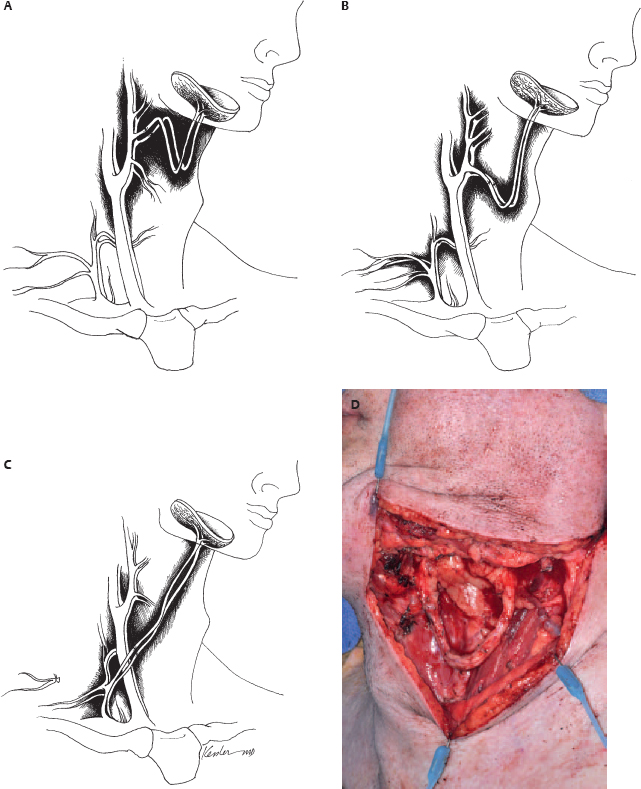
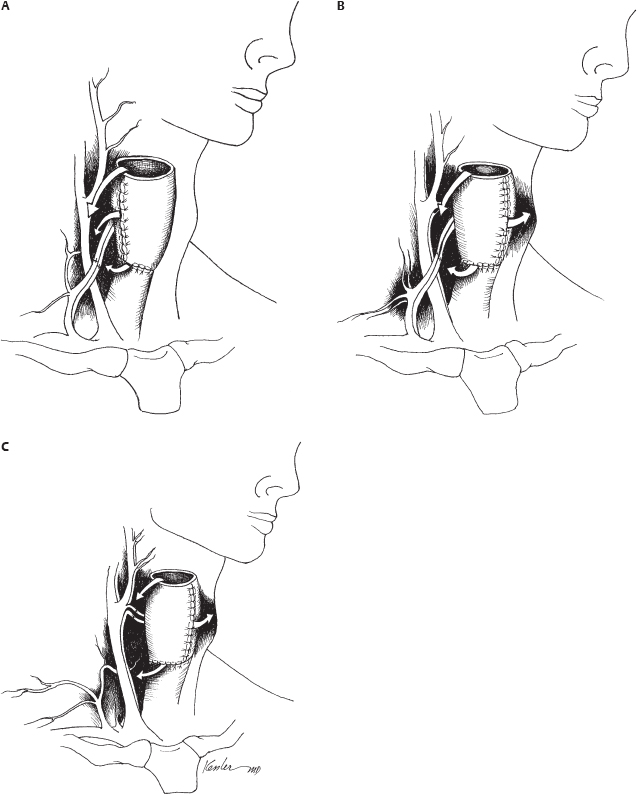
10 Microvascular reconstruction of the head and neck continues to challenge surgeons worldwide despite significant technical advances. Paramount to successful microvascular reconstructive surgery is appropriate management of the microvascular anastomosis and vascular pedicle. The details of vessel management and microvascular anastomosis are critical to surgical success and are often ignored. This chapter discusses the general considerations and technical details, and provides a framework for successful vessel management for microvascular free tissue transfer in the head and neck. The vascular anatomy of the neck is well described, and a complete review of the anatomy is beyond the scope of this chapter. Nevertheless, microvascular surgeons have multiple vascular donor options within the head and neck for microvascular surgery. Essentially, arterial donor vessels may be divided into two categories: branches of the external carotid and branches of the thyrocervical trunk (Fig. 10.1). Although major branches of the external carotid artery such as the facial and the superior thyroid provide the majority of recipient vessels in microvascular head and neck reconstruction, anatomic issues, vessel availability, and the technical aspects of the reconstruction often preclude the selection of these vessels. In these situations, and in the situation of the vesseldepleted neck, selection of vessels may require accessing the thyrocervical trunk or branches of the external carotid less commonly utilized by reconstructive surgeons. It is in situations such as these that confusion or poor vessel selection and orientation may occur, resulting in a failed reconstruction. Therefore, understanding the anatomy of the vasculature of the head and neck in the context of the reconstructive goals is paramount for successful free tissue transfer. With these goals in mind, it is helpful to consider the vasculature of the head and neck in terms of arterial recipient vessel regions or zones. Zone I represents the superiormost region, including vessels available from the facial artery as it passes lateral to the mandible and superior to this level. Zone II represents the region of available cervical vessels below the mandible and contains the remaining branches of the external carotid, the most inferior being the superior thyroid artery. Zone III represents the most inferior region of recipient vessels, which includes branches of the thyrocervical trunk, thoracoacromial system, and internal mammary artery. It should be noted that these regions are meant to help reconstructive surgeons conceptualize the head and neck vasculature in an organized way, not to guide reconstructive decisions. The region of the planned reconstruction may or may not coincide with the Zone of recipient vessel selection. For example, a scalp reconstruction in Zone I may in fact also have recipient vessels in Zone I (i.e., superficial temporal artery/vein); however, a fibular reconstruction of the mandible (zone I) is likely to have recipient vessels selected within Zone II or III. In fact the majority of reconstructions often results in the selection of recipient vessels one or more zones removed from the reconstruction for the optimal pedicle configuration. Some detailed considerations of the recipient zones follow. Recipient vessels within Zone I include the facial artery lateral to the mandible, the ascending palatine artery, the angular artery (distal facial artery), the maxillary artery, and the superficial temporal artery. Access to deeper systems, such as the ascending palatine or maxillary artery, generally requires an ablative procedure that exposes these vessels, and they are infrequently utilized due to their anatomic location. Knowledge of the available vessel options within Zone I is critical for planning microvascular reconstructions of the nasal complex or anterior forehead/scalp and orbit. The facial artery and vein passing lateral to the body of the mandible provide excellent caliber and reliability within Zone I for microvascular reconstruction. Location of these vessels is readily achieved by palpation of the mandibular notch and careful dissection to identify the vessels for vascular access as well as to identify and protect the marginal branch of the facial nerve, which overlies the facial vein in this region. Distally, the angular/nasolabial branch of the facial artery may be reliably located within the nasolabial fold and has been used successfully for microvascular reconstruction.1 Cadaveric investigation revealed that the average length of the artery was 28 mm and the mean diameter of the respective artery and vein (1.5 and 2.5 mm) was suitable for microvascular anastomosis in 85% of the sides investigated.2 Successful intraoral preparation and microvascular anastomosis has been reported as well and represents an option for intraoral reconstructions when extraoral incisions may be avoided.3 Reconstructive surgeons have utilized the superficial temporal system of the external carotid artery for the reconstruction of facial, scalp, and maxillary defects.4,5 This system is often avoided or ignored by many surgeons due to unfamiliarity with the anatomy or concerns about vessel diameter and reliability. The location of the superficial temporal artery is extremely consistent and is approximately 1 cm anterior to the external ear and is readily located with Doppler examination. Advantages of this recipient site include avoiding previously radiated areas, good anatomic reliability, and the avoidance of vein grafting for reconstructions of this region. Dissection of the vessels often requires some dissection within the superior portion of the parotid gland, and careful attention to avoid damaging the frontal branch of the facial nerve is required. The superficial temporal vein is relatively thin, and careful dissection and avoiding excessive manipulation or kinking during microvascular anastomosis are required. Dissection should proceed immediately subcutaneously in this area until the superficial temporal vein is identified to avoid damaging the vein. Caution should be exercised when selecting these vessels for microvascular reconstruction if the region has received radiation.5 Recipient vessels located within Zone II are the most commonly utilized vessels for microvascular reconstruction of the head and neck. The branches of the external carotid artery (and in some cases the external carotid artery itself) provide excellent caliber and flow characteristics for microvascular reconstruction and have proven to be very reliable in large series of microvascular reconstructions. It should be noted that vessels within Zone II are often within the target region of previous radiotherapy for pharyngeal/laryngeal malignancies or metastatic cervical lymph nodes. Vessels that appear to have sufficient diameter may reveal significant intimal/medial thickening due to radiation, and the actual internal diameter may be quite attenuated under microscopic inspection. The reconstructive surgeon must verify adequate flow from the selected vessel prior to arterial anastomosis. As a general principle, selection of the artery with the strongest arterial flow rather than the largest diameter yields more reliable results. The facial artery is arguably the most commonly used vessel for head and neck microvascular reconstruction. Its favorable location, length, and diameter make it an ideal candidate for microvascular anastomosis within this region. Experienced microvascular surgeons have noted that the tethering of the facial artery by the digastric/stylohyoid muscles may preclude adequate access to the artery or introduce untoward positioning of the vascular pedicle. Division of the digastric muscle is recommended to address these issues.6 The lingual artery may be accessed in a similar fashion, and arises slightly inferior to the facial artery from the external carotid artery. Identification of the hypoglossal nerve in this area may be required to provide appropriate vascular access and avoid damaging the nerve. The superior thyroid artery provides excellent caliber and reliability for microvascular reconstructions. Cadaveric investigations have reported the outer diameter of the superior thyroid artery to be approximately 3.5 mm. It should also be noted that the reported location of the artery in relation to the carotid bifurcation is somewhat variable.7 The superior thyroid artery offers an additional advantage of having an inferior orientation relative to the superior orientation of all other branches of the external carotid. Excellent arterial length may be obtained by tracing the artery inferiorly until several branches supplying the thyroid gland are encountered and vessel diameter is compromised. Interestingly, using the superior thyroid artery in a reverse flow pattern has been reported in the microsurgical literature; however, the reliability of this technique has not been evaluated.8 Zone III represents the most inferior region of recipient vessels, which includes branches of the thyrocervical trunk, thoracoacromial system, and internal mammary artery. These vessels have been extensively utilized in situations in which Zone II vessels are unavailable or are in an unfavorable location related to the reconstruction. The thyrocervical trunk may be identified posterior to the sternal attachment of the sternocleidomastoid muscle deep to the clavicular attachments and deep to the omohyoid muscle. The vessels of the thyrocervical trunk including the inferior thyroid, superficial cervical, and suprascapular artery may be found within the cervical fat overlying the anterior scalene muscle. The deep cervical fascia overlying the anterior scalene muscle should be kept intact during surgical dissection to prevent damage to the phrenic nerve. Prior to arterial division within this region the surgeon should verify that a branching pattern exists on the proposed recipient artery as the vertebral artery arises slightly medial to the origin of the thyrocervical trunk from the subclavian artery and may inadvertently be damaged, with severe consequences. The thyrocervical system represents the ideal arterial system for microvascular surgeon in the vessel-depleted neck. It is important to note that the microvascular surgeon may wish to access the thyrocervical system for microvascular anastomosis merely to optimize pedicle orientation despite the availability of external carotid recipient vessels. The preoperative assessment of patients who are to undergo microvascular free tissue transfer is important to successful surgical outcomes. The impacts of medical comorbidities and of age, to some degree, are recognized by microvascular surgeons and frequently alter the management considerations when free tissue transfer techniques are employed.9–11 Previous radiation therapy has been reported to be a positive predictor for wound complications after microvascular reconstruction; however, the impact of these therapies continues to be investigated, and although an adverse effect may be suspected, debate regarding the actual effects of radiotherapy continues.12–14 The implications of body habitus and general anatomic factors are frequently ignored by inexperienced surgeons but may have a significant impact during free tissue reconstruction. Obesity, short neck, radiation fibrosis, and cervical osteoarthritis may impair the ability of the microsurgeon to harvest, inset, and orient the microvascular reconstruction in a favorable configuration.15 Tunneled vascular pedicles, which may be performed routinely in patients with normal body habitus, may represent significant technical challenges in obese patients, resulting in untoward twisting and stretching of the vascular pedicle. Knowledge and selection of appropriate flaps with long vascular pedicles to relieve tension, consideration of vein grafting, or altering operative approaches to improve access may address these issues. Review of previous operative reports can yield information related to the vasculature available for microvascular anastomosis. Additionally, operative details may offer insight into the difficulties that may be encountered when additional procedures are performed. Procedures such as neck dissections, thyroidectomy, submandibular gland surgery, tracheostomy, carotid endarterectomy, and previous cervical spine surgery via an anterior approach may not preclude the availability of a microvascular vessel but will undoubtedly have some level of impact on operative findings when performing free tissue transfer. Previous microvascular reconstruction will have an obvious impact, and operative reports related to previous free tissue transfers should be carefully reviewed. Although the focus of the microsurgeon includes the location of suitable vessels for microvascular reconstruction, often other technical issues as noted above dominate the surgical challenge and lead to complications postoperatively. Therefore, it is important for the reconstructive microsurgeon to have mastery of the anatomic considerations and a wide variety of reconstructive options available to address the multitude of challenges that may arise during surgery. More importantly, the microsurgeon must recognize the inherent factors present in each individual patient to allow for adequate preoperative planning and maximize success. Although the routine use of preoperative imaging in the surgical planning for ablative surgery is widely accepted, preoperative imaging obtained specifically for microvascular surgery is often unnecessary. There are, however, several situations in which imaging is indicated prior to reconstruction. Perhaps the most obvious indication for preoperative imaging is the assessment of peripheral vessels in fibular surgery in patients with longstanding peripheral vascular disease. Computed tomography (CT) or magnetic resonance angiography (MRA) (or formal flow Doppler investigation) should be considered to evaluate the lower extremity vasculature in patients with appropriate risk factors undergoing fibular free transfer.16–20 The routine use of angiographic studies for the detection of peroneal artery septocutaneous perforators continues to be evaluated; however, it is probably unnecessary in the majority of cases.21,22 Routine angiography/Doppler evaluation of the cervical vasculature is often unnecessary, but it should be considered in cases with multiple prior procedures, a history of severe vascular disease, multiple vascular/surgical insults, or chronic wounds.23–25 Regarding the radial forearm flap, preoperative imaging of the palmar arch is frequently not indicated, but a negative Allen’s testing does not preclude significant vascular disease of the palmar arch, and preoperative Doppler imaging may be considered if significant vasculopathy is suspected.23,24,26 There are several objectives that should be recognized by the microsurgeon for successful pedicle orientation. The most obvious are related to vascular compromise of the flap. The experienced microsurgeon makes every effort to recognize the potential factors leading to vascular compromise prior to performing microvascular anastomosis. Repositioning transferred tissues and the vascular pedicle is infinitely more difficult, if not impossible, if the possibility of compromise is recognized after the flap inset and microvascular anastomosis has been performed. Avoiding vascular pedicle compression related to anatomic factors, flap orientation, and skin closure is relatively obvious but can be difficult to achieve if the potential for compression is not recognized early during reconstruction. Similarly, it is important to avoid a kink or twist in the vascular pedicle during flap positioning. Favorable pedicle geometry implies gentle pedicle curvature with alignment of the microvascular anastomosis (Fig. 10.2). Although external cutaneous monitors may be helpful in select cases, flap orientation complexity increases with their use and may compromise the geometry of the reconstruction. Perhaps less obvious is the avoidance of placing the microvascular anastomosis in positions of possible peril. These positions include pedicle placement adjacent to areas of possible pharyngeal anastomotic leak, tracheostomy sites, positions of external compression leading to vascular compromise, and positions immediately beneath areas of cutaneous vascular compromise, which may lead to anastomotic exposure (Fig. 10.3). Planning for double free tissue transfers requires further attention by the microsurgeon to avoid technical difficulties related to pedicle geometry and vessel availability. Avoiding unnecessary destruction of recipient vessels during tumor ablations is critical for successful reconstruction in these situations. Pedicle length and diameter match with proposed recipient sites should be planned prior to flap inset. Pedicle orientation issues, which remain unrecognized until the second tissue transfer is prepared for anastomosis, are problematic and may be avoided with appropriate planning. It should be noted that the previously radiated or operated neck does not preclude the use of recipient vessels from that side. Additionally, sequential or “piggyback” configurations should be avoided, as proximal anastomotic compromise may sacrifice both reconstructions.27,28 Similarly, configurations resulting in retrograde flow, although possible in the first vascular territory, are not generally recommended due to decreased vascularity and flap survival. Although this finding may seem intuitively obvious, many authors have reported retrograde anastomosis despite the evidence of the inherent risks associated with this technique.29 ♦ The primary goal of pedicle orientation is to avoid vascular compression and vessel kinking that results in vascular compromise; this goal take precedence over all other considerations. ♦ Careful attention to small cutaneous perforators is required to avoid compromise; harvesting small perforators with a muscle cuff is recommended if possible. ♦ Prior to performing microsurgical anastomosis, the microsurgeon verifies the position of the reconstructive tissue to optimize pedicle orientation and geometry. ♦ The order of microvascular anastomosis (arterial versus venous) may vary depending on the pedicle geometry to facilitate microsurgery. ♦ Avoid placing the anastomosis in positions of peril. ♦ Microvascular anastomosis should be performed to the highest flow vessels available that do not compromise pedicle geometry. ♦ Careful planning for double free tissue transfers will avoid unnecessary technical difficulties during microsurgery. ♦ The external cutaneous paddle for monitoring should not be employed at the expense of appropriate pedicle geometry. ♦ Avoid retrograde flow configurations if possible. The preparation of vessels prior to microsurgical anastomosis is a critical component of microsurgery, although it is often overlooked in the microsurgical literature. Preparation of both the recipient and donor artery should provide adequate vessel length for anastomosis without damaging the vessels. Careful attention to avoiding manipulation of the internal lumen and vessel intima to prevent damage to the endothelium is paramount to prevent arterial thrombosis. Recipient/donor veins are similarly prepared, although careful examination of the internal anatomy is required to avoid adjacent branches or valves, which may dispose the anastomosis to thrombosis. Careful vessel preparation improves visualization of the internal lumen of the vessels and allows the microsurgeon to prevent vessel wall overlap during anastomosis. Adventitia may interfere with knot tying and, of greater concern, be trapped within the lumen of the anastomosis in situations of inadequate vessel preparation. Additional vessel preparation may be required in special circumstances such as vein grafting, application of monitoring devices, or for certain vessel configurations. ♦ Microscopic/loupe visualization is critical; avoid vessel preparation/manipulation without magnification. ♦ Prepare the vessels adequately for anastomosis, and avoid aggressive vessel manipulation and manipulation of the internal lumen. ♦ Prepare sufficient vessel length to avoid adventitial interference and provide sufficient nontraumatized vessel length to facilitate microsurgery. ♦ Radiated/thickened vessels may require additional preparation to provide optimal vessel wall thickness for accurate anastomosis. ♦ Heparinized saline (100 U/mL) is used for irrigation and visualization and prevention of thrombosis during microsurgery.
Microvascular Reconstruction of the Head and Neck
♦ RELEVANT ANATOMY
Zone I
Zone II
Zone III
♦ PREOPERATIVE CONSIDERATIONS
Imaging Studies
Reconstructive Implications for Vessel Orientation
Surgical Technique and Considerations
Vessel Preparation Prior to Anastomosis
Surgical Technique and Considerations
MICROVASCULAR ANASTOMOSIS
Arterial Anastomosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree