Fig. 17.1
(a) AP, (b) lateral, and (c) merchant plain radiographic views demonstrating the location of the tibial and femoral tunnels and hardware fixation including a tibial metallic interference screw and a lateral cortical button fixation. Care should be taken to evaluate the degree of tunnel widening on these images
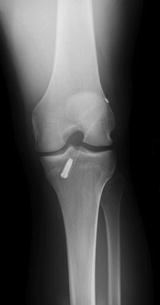
Fig. 17.2
Weight bearing 45° PA view plain radiograph demonstrating preservation of posterior joint space
MRI should be obtained to evaluate the status of the menisci, ligaments, cartilage, and subchondral bone. Additional cartilage-specific MRI sequences including three-dimensional fat suppression, proton density, and two-dimensional fast spin-echo can be used to fully assess patellofemoral and tibiofemoral hyaline cartilage and subchondral bone [23]. Cartilage signal intensity and morphology should be noted. Increased signal within the chondral layer, chondral fissuring, and subchondral sclerosis or edema may be identified and are suggestive of chondral damage (Fig. 17.3). Advanced, quantitative MR imaging protocols including measurement of T2 relaxation time (measure of collagen organization) and T1 rho (measure of proteoglycan content) are more sensitive for early degenerative changes in articular cartilage. Computed tomographic (CT) scan may be used to evaluate the bony architecture and facilitate preoperative planning including ACL graft tunnel placement. This planning is particularly important if the revising surgeon uses a bone plug technique for meniscal transplant, since multiple tibial tunnels will be required for fixation. While triple-phase bone scan is not routinely obtained in the setting of meniscal transplant in revision ACL reconstruction, increased uptake may suggest compartment overload and impending chondral damage, thus increasing the importance of attempting to restore the compartment load-sharing through MAT. Gait analysis is also rarely required but has been previously used to evaluate compartment overload in the setting of meniscal injury and concomitant malalignment [7]. Malalignment should be considered during preoperative planning, as correction of malalignment with a high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) may be required with or without a meniscal transplant. The patient’s response to a trial in an unloader brace provides helpful information about relative compartment overload.
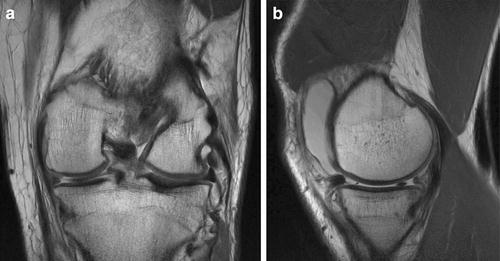

Fig. 17.3
MRI (a) coronal and (b) sagittal views demonstrating preserved cartilage with absent medial meniscus
Preoperative Considerations
Allograft Procurement and Processing
Meniscal allograft selection for MAT in revision ACL reconstruction requires consideration of graft procurement, processing, and storage, graft sizing, and timing for donor matching and implantation. Various meniscal allograft preservation options exist including fresh, fresh-frozen, cryopreserved, and lyophilized. Cellular viability is preserved in fresh and cryopreserved grafts. However, fresh allograft implantation is required within days of procurement to maintain cellular viability, which presents difficult logistics. In an effort to preserve cell viability while allowing increased storage time, cryopreservation techniques have been developed. These techniques use controlled-rate freezing with cryoprotectants such as glycerol to protect cell viability. However, only cells on the meniscus surface are protected, with little preservation of cells deep in the meniscus substance. Fresh-frozen tissue does not contain any viable cells, as the freezing process kills all cells. These tissues require cellular repopulation after transplantation, which occurs from synovial cell ingrowth. Fresh-frozen tissue can be stored indefinitely, allowing for elective surgical scheduling. Fresh-frozen tissue is the most commonly used allograft type [2, 24]. Lyophilized grafts are acellular and thus can be stored for prolonged periods, but have been associated with graft shrinkage. The current authors do not suggest using lyophilized grafts for this reason [25, 26].
The risk of disease transmission and immunogenicity has been considered for fresh and fresh-frozen allografts and allograft rejection has been documented [27]. Sterilization methods including gamma irradiation and ethylene oxide have previously been employed in an attempt to reduce the risk of disease transmission. Gamma irradiation will reduce or eliminate allograft viral DNA; however, a minimum radiation strength of 3.0 mrad is required for sterilization and this magnitude of irradiation has been associated with impaired meniscal tissue properties [28]. Ethylene oxide sterilization is effective in reducing disease transmission for lyophilized grafts but has been associated with synovitis due to the pro-inflammatory ethylene chlorohydrin byproduct [29].
Careful donor screening rather than graft processing procedures, however, most effectively decreases the risk of disease transmission. Meniscal allograft immunogenicity is also a consideration and is primarily associated with the cellular elements of the attached bone plugs or block. However, no animal studies have substantiated this concern [30–33]. Moreover, data from massive osteochondral allograft transplantation have documented a minimal rate of immunogenicity [27]. It is our opinion that the sterilization risks and minimal concern for immunogenicity outweigh the potential benefits and therefore the preferred option is the fresh-frozen, nonirradiated meniscal allograft.
Allograft Sizing
Meniscal allograft size matching represents a crucial component of preoperative planning and significant limiting factor in graft availability. Meticulous preoperative graft size matching helps optimize ease of implantation and meniscal allograft mechanical function [34]. Intraoperative and radiographic measurement methods may be used to ensure donor-recipient size matching. Radiographic measurements of the meniscus or tibial plateau are most commonly used. Plain radiograph, CT scan, and MRI may be used to calculate donor graft dimensions [35]. Significant controversy exists regarding the measurement accuracy among these modalities [35–37]. As a result, the most accurate, relevant anatomical landmarks for meniscal allograft measurement remain unclear. Previous authors have suggested MRI measurement of the contralateral, intact meniscus; however, significant intrasubject variability between contralateral menisci has been observed [35, 38]. Direct or radiographic measurement of the injured, ipsilateral meniscus is often impossible secondary to prior meniscectomy, especially in the revision setting.
Given the variability among soft tissue landmarks, the authors’ prefer to match size based on tibial dimensions. There is a reliable relationship between bony, radiographic landmarks and meniscus size [39, 40]. Nevertheless, this technique has also been associated with significant variability up to 8.4 % or 3.8 mm in meniscal length and width relative to the respective tibial plateau dimensions [39]. MRI matching data have documented improved correlation rates, but 65 % of the images differed by more than 2 mm of actual measured plateau dimensions [35]. While an exact donor-recipient size match is considered optimal, limited data exists regarding tolerance for size mismatch or the effects of meniscal allograft size mismatch [41]. The current authors’ use both plain radiographs with a size magnification marker and MRI to determine tibial plateau osseous measurements. These measurements allow donor matching for a fresh-frozen tibial plateau or hemiplateau with attached meniscal allograft(s).
Pollard et al. [39] described the technique used by the current authors for allograft donor size matching using plain radiographs. Lateral and anteroposterior radiographs corrected for magnification determine the meniscal length and width. The lengths of the lateral and medial menisci are calculated by multiplying the sagittal length of the tibial plateau on the lateral radiograph by 0.7 and 0.8, respectively. Correct use of this technique will provide a graft size mismatch risk of less than 5 % (Fig. 17.4).
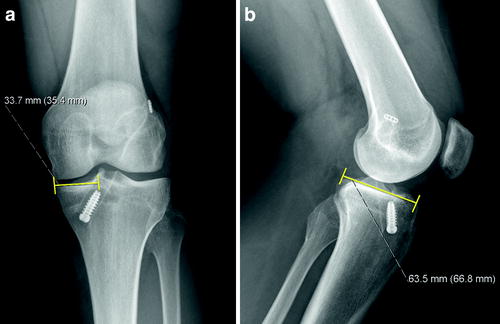

Fig. 17.4
(a) AP and (b) lateral plain radiographs demonstrating medial meniscal allograft sizing technique using the medial tibial plateau osseous dimensions
When submitting the aforementioned measurements, the surgeon should be familiar with the providing tissue bank, associated procurement techniques, and sizing restrictions. In some circumstances, the tibial plateau-meniscus graft may not be available. Rather, isolated meniscal allograft tissue may be the only available option. In this setting, a measurement formula specific to the meniscal soft tissue is required for size matching. Moreover, the graft should be checked prior to induction of anesthesia, due to the rare incidence of an unacceptable graft due to a tear or compromise of the bone.
Tunnel Placement
Tunnel placement is particularly important in the setting of MAT in revision ACL reconstruction. Prior tibial ACL tunnels with or without tunnel widening may require ACL tunnel repositioning, which can further reduce the space remaining for meniscal tunnel placement. Additional tunnels with a bone plug meniscal transplant technique may be difficult, especially with medial meniscal transplant due to the medial approach commonly used for ACL tibial tunnel placement. Careful preoperative evaluation with CT scan and MRI should be used to plan for tunnel placement or changing to a slot or “keyhole” technique (Fig. 17.5). The tunnel and keyhole positions should be considered not only at the level of the plateau but also within the tibial metaphysis. Nevertheless, tunnel compromise can occur even with the keyhole technique. In either technique, the surgeon must ensure that both the anterior and posterior meniscal horns are securely fixed [42–44]. If there are excessively enlarged tunnels, a two-stage approach might be considered, with bone grafting of the tunnels, followed by graft implantation after tunnel healing. Another option to consider in the setting of enlarged bone tunnels is a blind-ended tunnel in the tibia using a reverse cutting drill (Retrodrill, Arthrex™) (Fig. 17.6).
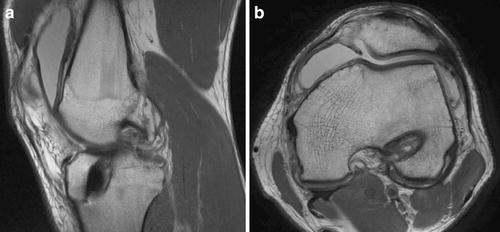
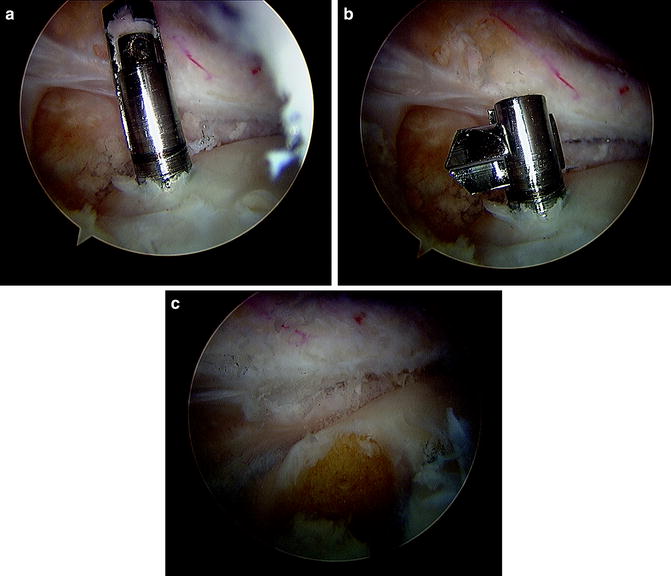

Fig. 17.5
(a) Sagittal and (b) axial MRI of tibial and femoral tunnel positions, respectively. Minimal evidence of tunnel widening is present in these images. CT imaging may be used to more effectively evaluate osseous architecture if necessary

Fig. 17.6
Arthroscopic view of the posterior, medial compartment. (a) The retrocutting drill (Retrodrill, Arthrex™) can be placed using an alignment guide in a standard guidepin fashion. (b) The cutting piece is deployed, and (c) the blind socket is then created using the reverse cutting technique
Surgical Technique
Compartment Preparation
Diagnostic arthroscopy should be performed prior to MAT in revision ACL reconstruction. Radiographic and clinical findings should be visually confirmed and chondral surfaces should be inspected to rule out advanced arthrosis. This inspection is particularly important if the index and revision surgeons differ.
Following diagnostic arthroscopy, the residual meniscal tissue in the desired transplant compartment should be debrided until punctate bleeding is encountered within 1–2 mm of the peripheral rim. Preservation of meniscal vasculature is an important factor in meniscal repair or transplantation. Vascular penetration of the peripheral 10–30 % of the medial meniscus and 10–25 % of the lateral meniscus occurs from the inferior medial and lateral geniculate arteries [45]. The blood supply from this plexus in addition to synovial fluid diffusion serves to provide nutrition to the menisci. Vessels from the capsular periphery provide a source for vascular ingrowth into the transplant.
The osseous insertions of the anterior and posterior horns should also be identified for anatomic transplant tunnel or slot placement. A sub-posterior cruciate ligament (PCL) medial femoral condylar notchplasty or sub-ACL lateral femoral condylar notchplasty may then be performed to provide increased visualization of the posterior horn insertion. This notchplasty will also allow improved passage of the bone plug or keyhole bridge. An open posteromedial or posterolateral approach should also be utilized in preparation for inside-out meniscal capsular suturing.
Allograft Preparation
Preparation of the meniscal allograft directly depends on the fixation method desired by the revising surgeon. This method should be determined during preoperative planning. Intraoperatively, the hemiplateau or full plateau with the attached meniscal allograft should be inspected prior to patient sedation to confirm tissue quality. Following confirmation, excess soft tissue should be removed from the plateau leaving only meniscal tissue and bone. The anterior and posterior horns should then be identified and marked with a sterile marker to ensure anatomic placement and minimize confusion. The hemiplateau can then be machined for either bone plug or keyhole fixation. Sutures are placed through the bone plugs to aid in graft placement and fixation. A traction suture should also be placed at the junction of the middle and posterior third of the meniscus. This suture serves to facilitate meniscal orientation and reduction during placement (Fig. 17.7).
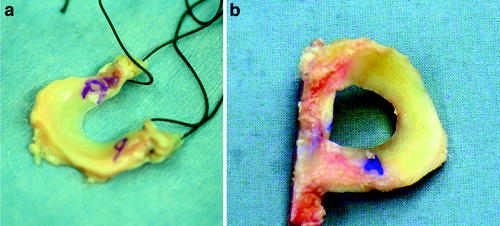

Fig. 17.7
Photograph demonstrating the prepared meniscal allograft including markings for anterior and posterior orientation, traction suture placement, and (a) fashioned bone plugs or (b) keyhole slot
Surgical Pearls
As mentioned previously, MAT in revision ACL reconstruction can be performed using a bone plug or keyhole technique. The decision regarding which technique to use is largely dependent upon surgeon preference and familiarity. Surgical technique specifics have been previously documented for both techniques and should be reviewed if necessary [3, 46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








