Fig. 8.1
The differences between mast cells and basophils. Mast cells and basophils have some differences, for instance, in their development, distribution, and proliferation
Although a number of functions of mast cells or basophils have been reported as mentioned above, the true in vivo functions of both cell types are still largely unknown due to the fact that these cells are rare and are difficult to isolate as pure populations. Furthermore, there have been no ideal tools to evaluate their in vivo role, such as conditional depletion systems. Recently, new types of genetically modified mouse strains have been developed, and previously proposed functions of mast cells and basophils have been revisited extensively. In this review, we summarize these recent studies of mast cells and basophils and focus on their role in cutaneous immune response.
8.2 Characteristics of Mast Cells
Mast cells are derived from hematopoietic stem cells, but they do not ordinarily circulate in their mature form [8]. Instead, the precursor cells differentiate and mature locally after their migration into the vascularized tissues in which mast cells will ultimately reside [8]. Mast cells are widely distributed throughout tissues, especially near surfaces exposed to the environment, such as the skin, airways, and gastrointestinal and genitourinary tracts, where pathogens, allergens, and other environmental agents are encountered [9]. This distribution permits these cells, along with dendritic cells (DCs) and tissue macrophages, to be among the first cells in the immune response system to interact with environmental antigens and allergens [10]. Mast cells can survive for a long time, and some tissue mast cells proliferate in situ in response to certain forms of stimulation [8] (Fig. 8.1). Recent study has shown that mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis [11].
Mast cells can participate in many cycles of activation for the release of mediators and can be activated to release distinct patterns of mediators or cytokines, depending on the type and strength of the activating stimuli [9]. Tumor necrosis factor (TNF)-α is a major cytokine stored and released by mast cells. It upregulates endothelial and epithelial adhesion molecules, increases bronchial responsiveness, and has antitumor effects. Other cytokines produced by mast cells include interleukin (IL)-3, granulocyte macrophage colony-stimulating factor (GM-CSF), and IL-5, all of which are critical for eosinophil development and survival, and IL-6, IL-10, and IL-13 [8, 9].
8.3 Characteristics of Basophils
Basophils are the least abundant leucocytes and are primarily found in the circulation system. They comprise only a small percentage (<0.5%) of circulating blood cells under steady-state conditions but rapidly expand in the bone marrow in response to inflammatory signals, whereupon they are mobilized to the blood, spleen, lungs, and liver. They are generated from the granulocyte–monocyte progenitors in the bone marrow and populate the periphery as fully mature cells [12]. The lifespan of basophils is short [13] (Fig. 8.1). For many years, basophils have been a somewhat enigmatic immune cell type and questions regarding their role in protective immunity as well as the specific pathogens or insults that elicit basophil responses are not fully answered. Studies of basophils have been hindered by the lack of information about how they can be distinguished both phenotypically and functionally from developmentally related mast cells.
Basophils can be activated by an array of signals, including those mediated by antibodies (IgE, IgG, and IgD), cytokines (IL-3, IL-18, and IL-33), proteases, Toll-like receptor (TLR) ligands, and complement factors [12, 14]. Activated basophils are capable of producing a variety of secreted factors including the cytokines IL-4, IL-13, IL-6, TNF-α, and thymic stromal lymphopoietin (TSLP), preformed mediators such as histamine and leukotriene C4 (LTC4), antimicrobial peptides, and chemotactic factors known to recruit multiple immune cell types [15, 16].
8.4 Mast Cell-Specific Depletion Model
The selective deletion of mast cells or basophils in vivo is a useful approach through which to address the role of these cell types during immune responses. Classic models used to study mast cell functions are based on Kit-mutant mouse strains. In addition to their mast cell defect, Kit W/W v mice have multiple hematopoietic abnormalities that include compromised fitness of the hematopoietic stem and progenitor cells [17], severe macrocytic anemia [18], impaired T development in the thymus [18], and a shift in intraepithelial T cells in the gut in favor of T cell receptor (TCR) αβ+ cells and against TCR γδ+ cells [19]. It is important to note that this Kit mutant is neutropenic, which may be a major factor affecting immune responses in this strain [20]. New mouse models have recently been developed to avoid these problems. Several groups have reported the generation of mice expressing Cre recombinase under the control of mast cell protease genes [21–23], and, in 2011, four laboratories used their mice or additionally generated lines to obtain Kit-independent mast-cell–deficient mouse strains [6, 24–26]. Some groups have used the diphtheria toxin (DT) system for depletion of the mast cell lineage [6, 24] and other groups have ablated mast cells constitutively by exploiting the genotoxicity of Cre-recombinase [25]. Furthermore, another group depleted mast cells by the Cre-mediated deletion of an apoptosis suppressor gene [26]. Strain-specific characteristics are summarized in Table 8.1.
Table 8.1
Mast cell and basophil depleted model
Mouse strain | Description | Model of depletion | Deleted Population | Remarks | Refs |
|---|---|---|---|---|---|
Mast cells | |||||
Mcpt5-Cre | BAC transgene (129 kb, Cre inserted after the Mcpt5 start codon) | Cross to Cre-iDTR mice or R-DTA mice | CTMCs (>90 %) | MMCs and basophils are not deleted | |
Cpa3-Cre | Promoter transgene | Mcl-1fl/fl mice causes impaired survival | CTMCs and MMCs (>90 %), basophils (60–80 %) | Mice develop splenic neutrophilia and macrocytic anemia | [26] |
Chm:Cre | Promoter transgene (600 bp baboon α-chymase promoter) | NR | NA | Marks mast cells in the lung and the colon | [22] |
Cpa3Cre | Knock-in of Cre before the first exon of Cpa3 | Constitutive depletion | CTMCs and MMCs (100 %), basophils (60 % in the spleen) | NA | [25] |
Mas-TRECK | DTR transgene (under control of 5′ enhancer, promoter, and intronic enhancer of IL4) | DT injection | CTMCs, MMCs, and basophils (90–100 %) | Basophils are restored 2 weeks after DT treatment | |
Basophils | |||||
Basoph8 | Knock-in of IRES-YFP-Cre cassette before the Mcpt8 start codon | Cross to R-DTA mice | Basophils (>90 %) | NA | [28] |
Mcpt8-Cre | BAC transgene (228 kb, Cre inserted after the Mcpt8 start codon) | Constitutive depletion | Basophils (>90 %) | NA | [29] |
Mcpt8DTR | Knock-in of IRES-DTR-EGFP cassette in 3′ UTR of Mcpt8 | DT injection | Basophils (>90 %) | NA | [30] |
P1-Runx1 | Knockout | P1-Runx1 seems to be important for the basophil lineage | Basophils (>90 %) | NA | [31] |
Bas-TRECK | DTR transgene (under control of 5′ enhancer, promoter, and intronic enhancer of IL4) | DT injection | Basophils (>90 %) | NA | [27] |
8.5 Basophil-Specific Depletion Model
Due to the lack of any natural mouse mutants with basophil deficiencies, antibodies that deplete this population of cells have often been used to study the contribution of basophils in different experimental settings. These antibodies recognize either FcεRI (clone MAR-1) or the orphan activating receptor CD200 receptor 3 (CD200R3 ; clone Ba103), which are both mainly expressed by basophils and mast cells. Although both antibody clones can efficiently deplete basophils, they can also activate mast cells [29, 32]. Furthermore, the depletion of basophils by Ba103 is FcR-dependent and might therefore activate myeloid cells and natural killer (NK) cells [33]. MAR-1 also depletes a subset of FcεRI-expressing DCs [34]. Several in vivo functions have been attributed to basophils on the basis of studies using these depleting antibodies and this has led to several conclusions: first, that basophils cause IgG1-mediated systemic anaphylaxis [35], second, that they contribute to protective immunity against Trichuris muris [36], and third, that they enhance humoral memory responses in the spleen [37]. Several new mouse strains with a constitutive or inducible depletion of basophils have recently been generated, and studies using these mice could confirm some of the previously proposed effector functions of basophils.
8.6 Mast Cell and/or Basophil Involvement in Several Skin Diseases
Various models of severe inflammatory autoimmune diseases reveal that neutrophil infiltration into sites of local inflammation and tissue destruction critically depend on mast cells. Involvement of mast cells has been suggested in several animal disease models, such as psoriasis, rheumatoid arthritis (RA) [38–40], and bacterial infection [41, 42]. These observations seem to be highly relevant in terms of our understanding of human diseases, as large numbers of activated mast cells infiltrate the tissues in the corresponding human diseases, such as allergic contact dermatitis, psoriasis, and RA [43–45]. In addition to these diseases, mast cells are involved in multiple inflammatory and malignant diseases (Table 8.2). On the other hand, it is still largely unclear in which skin diseases basophils are involved. Recent studies have shown infiltration of basophils in several skin diseases, such as atopic dermatitis (AD), prurigo, and urticaria [46]. It is notable that skin lesions of bullous pemphigoid, classical eosinophilic pustular folliculitis (Ofuji’s disease), and Henoch–Schönlein purpura also frequently show tissue basophilia [46–49] (Table 8.3).
Table 8.2
Dermatological diseases with evidence for mast cell involvement
Anaphylaxis |
Hay fever |
Urticaria |
Localized mastocytomas |
Disseminated mastocytosis |
Mast cell leukemia |
Contact dermatitis |
Psoriasis and psoriasis arthritis |
Atopic dermatitis |
Bullous autoimmune diseases (bullous pemphigoid) |
Autoimmune vasculitis |
Systemic lupus erythematodes |
Systemic sclerosis and morphea |
Chronic graft-versus-host disease |
Morbus Morbihan and rosacea |
Skin infections |
Bacteria, fungi |
Parasites (Leishmania major) |
Skin tumors (basal cell carcinoma, spinocellular carcinoma, angiosarcoma) |
Table 8.3
Dermatological diseases with evidence for basophil infiltration
Atopic dermatitis |
Prurigo |
Urticaria |
Pemphigus vulgaris |
Bullous vulgaris |
Drug eruption |
Henoch–Schönlein purpura |
Insect bite (tick bite) |
Scabies |
Dermatomyositis |
Eosinophilic pustular folliculitis |
Leprosy (LL type) |
8.7 The Role of Mast Cells During Contact Hypersensitivity
Contact hypersensitivity (CHS) has been widely used as a model to study cutaneous immune responses, inasmuch as it is a prototype of delayed-type hypersensitivity mediated by antigen-specific T cells [50, 51]. CHS is classified as the sensitization phase and the elicitation phase. An essential step in the sensitization phase for CHS is the migration of hapten-bearing cutaneous DCs, such as epidermal Langerhans cells (LCs) and dermal DCs, into the skin-draining lymph nodes (LNs). After completing their maturation, mature DCs present antigens to naive T cells in the LNs, thus establishing the sensitization phase. In the subsequent challenge phase, re-exposure to the cognate hapten results in the recruitment of antigen-specific T cells and other non-antigen–specific leukocytes.
Mast cells are a candidate DC modulator because they express and release a wide variety of intermediaries, such as histamine, TNF-α , and lipid mediators. It has been reported that activated human cord-blood–derived mast cells induce DC maturation in vitro [52], that IgE-stimulated mast-cells–derived histamine induces murine LC migration in vivo [53], and that MC-derived TNF-α promotes cutaneous murine DC migration in vivo in an IgE-independent manner [54]. On the other hand, prostaglandin (PG) D2 is abundantly produced by mast cells in response to allergens [55] and inhibits LC migration [56]. Therefore, MCs might have bidirectional effects on DC activity in a context-dependent manner and the question of the mechanisms by which DCs are modulated by mast cells is an important issue to pursue.
Although basophils operate irrespectively of the development of CHS [7], the role of mast cells in CHS remains controversial. In some studies, mast-cell–deficient mice have exhibited reduced inflammation in TNCB-induced CHS [57, 58]. Other studies reported undiminished CHS induced with TNCB or DNFB [59, 60]. Furthermore, a recent publication reported that mast cells have regulatory roles through their production of IL-10, as mast-cell–deficient mice exhibited enhanced urushiol and DNFB-induced CHS [61]. In these studies, however, mice carrying mutations in the stem cell factor or its receptor c-Kit were used as mast-cell–deficient mice (C57BL/6-Kit W−sh/W−sh or WBB6F1-Kit w/w−v ). Although these mice lack mast cells, they also have various other immunological alterations, making it difficult to form a conclusion regarding the role of mast cells in CHS based solely on studies using these mice.
In new mast cell depletion models [7, 24], it has been reported that mice depleted of mast cells exhibited reduced CHS induced with FITC, Oxazolone , or DNFB [7, 24]. In addition, mast-cell–specific deletion of IL-10 did not result in exacerbated CHS. Without mast cells, skin DC migration and/or maturation and T-cell priming in the sensitization phase were impaired. Mast cells stimulated DCs via ICAM-1 or lymphocyte function-associated antigen one interaction and by membrane-bound tumor necrosis factor α on mast cells (Fig. 8.2). Interestingly, activated DCs in turn increased Ca2+ influx in mast cells, suggesting that mast cells and DCs interact to activate each other. In the elicitation phase, mast cell deficiency resulted in an impaired CHS response, probably as a result of reduced vascular permeability caused by a loss of histamine release from mast cells [24].
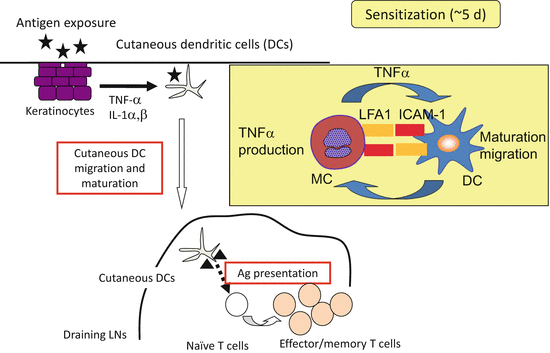

Fig. 8.2
Schema of contact dermatitis. During sensitization phases, mast cells bind to DCs in the dermis and stimulate DCs via ICAM-1 or lymphocyte-function–associated antigen one interaction as well as membrane-bound tumor necrosis factor α on mast cells
To date, it remains unknown why there is such a discrepancy between the reports using stem cell factor-deficient or c-Kit deficient models and those using conditional mast cell ablation models. One of the differences between these two models is the existence of melanocytes and hematopoietic stem cells. Recently, melanocytes were shown to express TLRs to modulate immune responses and to produce IL-1α and IL-1β [62, 63]. In addition, because of the congenital absence of mast cells in Kit W/Wv and Kit W−sh/W−sh mice, a compensatory mechanism may exist such as the repopulation of the skin with basophils [64]. Therefore, Kit W/Wv and Kit W−sh/W−sh mice may not necessarily be appropriate to evaluate the exclusive roles of mast cells.
8.8 The Role of Mast Cells During AD Pathogenesis
AD is characterized by skin inflammation, impaired skin barrier function, and IgE-mediated sensitization to food and environmental allergens. The etiology of this disease is not yet understood completely, but it is multifactorial; the disease, moreover, is characterized by complex interactions between genetic and environmental factors. Recently, two major hypotheses have come to the fore as possible explanations for the pathogenesis of this heterogeneous disease: (I) one assumes that the primary defect is an immune dysregulation that causes Th2-predominant inflammation and IgE-mediated sensitization [65]. In the other hypothesis (II), an intrinsic defect in the skin barrier function such as a filaggrin mutation is underscored as a primary cause of the disease [66, 67].
As most studies have shown increased numbers of mast cells in skin lesions in the AD models, it is generally assumed that mast cells contribute to skin inflammation. However, few studies have directly addressed whether, to what extent, or by what mechanism, mast cells play a role in the development of AD-like skin lesions. A cutaneous ovalbumin (OVA) patch model showed that skin inflammation is comparable in wild-type and Kit W/W−v mice [68]. On the other hand, skin inflammation induced by cutaneous sensitization with cedar pollen antigens was abolished in Kit W/W−v and Kit Sl/Sl−d mice [69]. However, mast cell reconstitution experiments have not been performed in either study. Interestingly, a recent study showed that FcɛRI and FcRγ are involved in a cutaneous OVA patch model [70]. Analysis using new cell-specific depletion models may answer this question.
8.9 New Role of Basophils During Th2 Skewing
The induction of Th2 immune responses was previously considered to depend mainly on DCs [71]. However, this dogma has recently been challenged because basophils might also play a pivotal role in this process [36, 72, 73]. It has been reported that CD49b+ FcεRI+ c-Kit− basophils migrate into draining LNs from the site of helminth infection or papain injection and thus act as APCs by taking up and processing antigens [36, 72, 73]. In addition, basophils express MHC class II and costimulatory molecules and secrete IL-4 and thymic stromal lymphopoietin (TSLP) , which are critical for Th2 development. Therefore, basophils alone are considered to induce Th2 polarization from naïve T cells without requiring DCs under certain conditions. In contrast, another group has found that IL-4-producing basophils were recruited to the mediastinal LNs upon primary exposure to house dust mites. In this case, they contributed to the strength of the Th2 response in the lungs but, in this model, basophils could not present antigens or express the chaperones involved in antigen presentation [34]. Therefore, the authors claimed that DCs were necessary and sufficient for inducing Th2 immunity to house dust mites in the lungs without the requirement of basophils. It has consistently been reported that Th2 responses are severely impaired either after Schistosoma mansoni egg injection or during active Schistosoma. mansoni infection by the depletion of CD11c+ cells but not by the depletion of basophils using anti-FcεRIα antibody [74]. Therefore, the role of basophils in the development of the Th2 response has been controversial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








