References
1. Carty NJ, Moran B, Johnson CD. Anorectal physiology measurements are of no value in clinical practice. True or false? Ann R Coll Surg Engl. 1994;76:276–80.
2. Vaizey CJ, Kamm MA. Prospective assessment of the clinical value of anorectal investigations. Digestion. 2000;61:207–14.
3. Eckardt VF, Elmer T. Reliability of anal pressure measurements. Dis Colon Rectum. 1991;34:772–7.
4. Glasgow SC, Birnbaum EH, Kodner IJ, Fleshman JW, Dietz DW. Preoperative anal manometry predicts continence after perineal proctectomy for rectal prolapse. Dis Colon Rectum. 2006;49:1052–8.
5. Pescatori M, Maria G, Anastasio G. “Spasm related” internal sphincterotomy in treatment of anal fissure. A randomized prospective study. Coloproctology. 1991;1:20–2.
6. Renzi A, Izzo D, DiSarno G, Talento P, Torelli F, Izzo G, et al. Clinical, manometric and ultrasonographic results of pneumatic balloon dilatation vs. Lateral internal sphincterotomy for chronic anal fissure: a prospective, randomized, controlled trial. Dis Colon Rectum. 2008;51:121–7.
7. Swash M. Henry MM. Coloproctology and the pelvic floor. Oxford: Butterworth Heinemann Ltd; 1992.
8. Miller R, Bartolo DC, Roe AM, Mortensen NJ. Assessment of microtransducers in anorectal manometry. Br J Surg. 1988;75:40–3.
9. Connell AM, McCall J, Misiewicz J, Rowlands E. Observation of the clinical use of radiopills. Br Med J. 1963;ii:771–4.
10. Browning C, Valori R, Wingate DL, Maclachlan D. A new pressure sensitive ingestible radiotelemetric capsule. Lancet. 1981;ii:509–15.
11. Womack NR, Williams NS, Holmfield JHM, Morrison JFB, Simpkins KC. New method for the dynamic assessment of anorectal function in constipation. Br J Surg. 1985;72:994–8.
12. Miller R, Lewis GT, Bartolo DCC, Cervero F, Mortensen NJ. Sensory discrimination and dynamic activity in the anorectum: evidence using a new ambulatory technique. Br J Surg. 1988;75:1003–7.
13. Kumar D, Waldron D, Williams NS, Browning C, Hutton MRE, Wingate DL. Prolonged anorectal monitoring and external anal sphincter electromyography in ambulant human subjects. Dig Dis Sci. 1990;35:641–8.
14. Sun WN, Read NW, Miner PB, Kurgan DD, Donnelly TC. The role of transient internal anal sphincter relaxation in faecal incontinence. Int J Colorectal Dis. 1997;12:296–7.
15. Dent JA. A new technique for continuous sphincter pressure measurement. Gastroenterology. 1976;71:263–7.
16. Orrom WJ, Williams JG, Rothenberger DA, Wong WD. Portable anorectal manometry. Br J Surg. 1990;77:876–7.
17. Bollard RC, Gardiner A, Duthie GS. Outpatient hand held manometry: comparison of techniques. Colorectal Dis. 2001;3:13–6.
18. Duthie GS. Ambulatory monitoring in anorectal disease [Thesis]. University of Aberdeen, Aberdeen, UK; 1992.
19. Szurszewski JH. A migrating electric complex of the canine small intestine. Am J Physiol. 1969;217:1757–63.
20. Kumar D, Williams NS, Waldron D, Wingate DL. Prolonged manometric recording of anorectal motor activity in ambulant human subjects: evidence of periodic activity. Gut. 1989;30:1007–11.
21. Wilgan P, Mishkinpour H, Becker M. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology. 1988;94:1105–6.
22. Ferrara A, Pemberton JH, Grotz RL, Hanson RB. Prolonged ambulatory recording of anorectal motility in patients with slow-transit constipation. Am J Surg. 1994;167:73–9.
23. Miller R, Orrom WJ, Duthie GS, Bartolo DCC, Mortensen NJM. Ambulatory anorectal physiology in patients following restorative proctocolectomy for ulcerative colitis and familial polyposis: a comparison of three reservoir designs. Br J Surg. 1985;72:470–4.
24. Romanos J, Stebbing JF, Smilgin Humphreys MM, Takeuchi N, Mortensen NJ. Ambulatory manometric examination in patients with colonic J pouch and in normal controls. Br J Surg. 1996;83:1744–6.
25. Ho Y-H, Tan M, Leong AFPK, Sedow-Choen F. Ambulatory manometry in patients with colonic J-pouch and straight coloanal anastomosis. Randomized controlled trial. Dis Colon Rectum. 2000;43:793–9.
26. Ho YH, Tan M. Ambulatory anorectal manometric findings in patients before and after haemorrhoidectomy. Int J Colorectal Dis. 1997;12:296–7.
27. Ronholt C, Rasmussen OO, Christiansen J. Ambulatory manometric recording of anorectal activity. Dis Colon Rectum. 1999;42:1551–9.
Manometric Parameters
Parameters measured with manometry are anal muscular tone, rectal compliance, anorectal sensation, and the RAIR. In our opinion, the report should not only provide measurement of anorectal pressures but must include all information available to the physician performing the examination (e.g., the inability to selectively contract the EAS with evidence of muscular agonist or antagonist synergies). Another parameter to evaluate while performing manometry is the perineal defence reflex. This physical parameter comprises the assessment of the pelvic floor and the abdominal musculature action after an intra-abdominal pressure increase [6]. The patient is asked to cough so that the physician can notice the perineal muscle contraction, resulting either in a physiological increase (reflex present) or a pathological descent (reflex absent) that, if marked, can be associated with emission of urine and flatulence. Manometrically it consists of an increase in anal pressure with cough. It has been known that normal values vary between the sexes and with age, depending on the examination modality and patient compliance; therefore, an interpretation of the examination in its totality, including the overall idea of anorectal function, is obtained [7]. Table 6.1 shows a typical report used in our outpatient unit.
Table 6.1
Typical report used in outpatient unit
Parameter Evaluated | Results | Normal Values (n.v) | |
Length of anal canal (high-pressure zone) | 4 cm | ||
Resting pressure (ARP) | 72 mmHg | n.v. 60–70 mmHg | |
Maximal voluntary contraction (MCV): amplitude | Increase 60 mmHg | n.v. >117 mmHg | |
Maximal volountary contraction (MCV): duration | 20 s | n.v (15–25 s) | |
Rectoanal inhibitory reflex (RAIR) with insufflation: | Present | ||
Threshold volume | 30 cc | ||
Time of relaxation (RT) | 14 s | ||
Time for recover of basal tone | 12 s | ||
Total | 26 s | ||
Rectal volumes | |||
Threshold of sensation | 30 cc | (n.v. 25–30 cc) | |
Threshold of stimulus | 60 cc | (n.v. 40–60 cc) | |
Maximum tolerable volume | 120 cc | (n.v. 80–160 cc) | |
Anal Muscular Tone
The highest pressure of a pull-through profile is defined as the maximal resting anal pressure, also referred to as the mean basal pressure (MBP), where normal values are poorly defined because of a variety of different techniques used and where they have been reported for only a small control population with a large expected range. The high-pressure zone corresponds anatomically to the condensation of the smooth-muscle fibers of the IAS and is shorter in women (2.0–3.0 cm) compared with men (2.5–3.5 cm) [8]. We consider normal MBP values to range from 60 to 70 mmHg for men and from 50 to 60 mmHg for women, with a maximal resting pressure located 1.5 cm from anal verge. In the composition of the mean anal canal resting tone, the IAS is responsible for 50–85 %, the EAS accounts for 25–30 %, and the remaining 15 % is attributed to expansion of the anal cushions. As a smooth muscle in a state of continuous maximal contraction and because of both intrinsic myogenic and extrinsic autonomic neurogenic properties, the IAS represents a natural defense to involuntary loss of stool. Although the IAS relaxes in response to rectal distension, it gradually reacquires tone as the rectum accommodates. Resting pressure in the anal canal exhibits regular fluctuations that vary during the day and at night on the basis of the presence or absence of fecal material in the rectum and posture, exhibiting longitudinal and radial variations. This functional asymmetry is found for both resting and squeeze pressure profiles and follows the inherent anatomic asymmetry in the arrangement of the sphincter muscles. In the proximal part of the anal canal, the recorded pressure is higher in the dorsal segment than in the anterior segment. This finding has been ascribed to the activity of the puborectalis muscle. In the mid-anal canal, the pressure is equally distributed in all segments, whereas in the lower anal canal the pressure is higher anteriorly. These differential pressure gradients have been implicated in the maintenance of the normal continence mechanism.
Voluntary Contraction
Voluntary contraction of the EAS produces an increase in anal pressure, superimposed on the basal tone. This increase in pressure usually escalates to two or three times the baseline resting tone (100–180 mmHg) and is maximal in the distal part of the anal canal, where the bulk of the EAS is situated. Because of muscular fatigue, maximal voluntary contraction of the EAS can be sustained for only 40–60 s. Maximal squeeze anal pressure (MSP) is higher in men than in women and is reduced as the subject gets older. Age-related reduction is more significant in women. The squeeze pressures are largely related to the contraction of the EAS, although the levator ani muscles also contribute. The fatigue rate index has been proposed as a manometric parameter to evaluate the voluntary component of anal sphincter function [9]. This parameter is calculated as the time necessary for the sphincter to be completely fatigued to a pressure equivalent to the resting tone. In our experience, the mean fatigue rate index is 3.3 min for volunteers, 2.8 min for constipated patients, 2.3 min for patients with seepage, and 1.5 min for incontinent patients. In fact, in some incontinent patients, despite an initial normal squeeze pressure, a rapid decrease in values can be noted; this assists in the discrimination of cases where endosonography is reported as either normal or equivocal and where there may be inherent EAS atrophy, pudendal neuropathy, or both. In a recent study, Brusciano et al. [10] evaluated muscular fatigue (in the context of the pucococcygeal test, although is not a manometric parameter) as a useful factor in the assessment of patients with defecatory disorders who may be treated with pelvic floor rehabilitation.
The EAS, along with the pelvic floor muscles, has three types of activity, namely, resting tone, reflex activity, and voluntary contractions [11]. Unlike other skeletal muscles, which usually are inactive at rest, the sphincter maintains continuous unconscious resting electrical tone as part of a reflex situated at the cauda equina level. In response to conditions of threatened continence (such as increased intra-abdominal pressure or rectal distension), the EAS and the puborectalis reflexly or voluntarily contract further to prevent leakage [12].
Comment: The Importance of the Anal Cushions in Anal Closure
Andrew P. Zbar
The anal cushion region is a mix of the supporting connective and elastic tissue of the hemorrhoid-bearing anal mucosa and submucosal elements positioned above the dentate line [1]. The contribution of the EAS has been formulated using simultaneous recordings of EAS electromyographic activity during anal pressure measurement in an effort to isolate IAS from EAS activity in both conscious and curarized patients undergoing general anesthesia [2], with an assessment of the contribution of the anal cushions by the manometric determination of a linear relationship between sphincter tension and anal canal diameter utilizing progressively larger perfused catheters for pressure determination and extrapolation of this relationship to the abscissa, where tension is zero [1, 3–5]. These assumptions are of no little importance because the anal cushion–bearing area is thought to provide an important mechanism of continence defense, the recent bolstering of which by deployment of synthetic bioaugmentable agents, including collagen, glutaraldehyde, silicone elastomer, carbon particles, and autologous fat, or the support provided by local radiofrequency application has been used with short- to medium-term success in cases of fecal incontinence where IAS damage has been considered to be the principal cause of reported incontinence. The subject of IAS bioaugmentation is covered in Chap. 33.
The issue of resting anal tone is still more complicated than this simple subcategorization. Manometric recording catheters have shown a gradual increase in pressure from proximal to distal in the anal canal, with the highest resting pressures usually recorded 1–2 cm rostral to the anal verge. This effective high-pressure zone corresponds anatomically to a condensation of smooth-muscle fibers, although it is not in the strictest sense a truly recognizable histological sphincter [6]. In this respect, it has been suggested that higher sectorial pressures recorded posteriorly and more cranially in the anal canal using three-dimensional software are reflective of part of the activity of the puborectalis muscle and are indicative of an overall thicker IAS, whereas higher distal resting pressures are recorded anteriorly, perhaps resulting from the disposition of the more distal superficial and subcutaneous components of the EAS [7]. It would seem that these initial views regarding the radial and longitudinal asymmetry of the sphincters, as a reflection of their anatomic distribution, to explain the continence mechanism are overly simplistic [8] and are not supported by histologic [9] or anatomic distribution of the sphincters [10], which is provided by more sophisticated and dedicated imaging: either three-dimensionally reconstructed endoluminal ultrasonography or surface phased-array magnetic resonance imaging [11].
For most purposes, maximal voluntary squeeze is attributed to the action of the EAS. During squeeze effort, recordings of such pressure (particularly after sphincter repair for incontinence) must be viewed with some caution because reproducible values are operator-dependent and, given the nature of inherent EAS squeeze, should be stated with specifics about whether squeeze was sustained or whether repeated measurements were made. Moreover, the adequacy of squeeze relies on the technician’s assessment of whether additional activities such as buttock clenching and thigh contraction were used as part of the activity. This fatigue index represents a voluntary pressure profile that may be recorded in the presence of initially normal squeeze pressures and perhaps is a more useful guide in those with incontinence correlating with continence symptom severity [12, 13]. This anatomic disposition of the sphincters and the puborectalis led to the “triple-loop” theory of continence, where Shafik postulated that the deep (upper) component of the EAS pulled the anal canal forward by virtue of its attachment to the lower border of the inferior pubic ramus, the middle EAS section drew the anal canal backward by its coccygeal attachments,and the subcutaneous EAS anchored the anal canal (and verge) anteriorly by complex attachments to the perianal skin [14]. This view was generally canonized in the physiologic literature by Dalley [15] and became the standard textbook mechanism of the maintenance of continence, and the IAS was regarded as of little clinical importance. On the basis of electromyographic studies during simulated defecation, Shafik et al. [16] further proposed that the levator ani contracted and shortened, both elevating and laterally displacing its normal position – a finding that has been disproven using dynamic computed tomography and magentic resonance defecography; actually, the reverse configuration of the levator ensues [17–21].
Traditionally, the IAS as a continence mechanism was disregarded, with most suggesting that the EAS could be partially divided (as often occurred during anal fistulectomy) without a continence-related consequence [22–26]. This data proved unfounded based on the recognition of often subtle impairments of continence that affect quality of life after internal anal sphincterotomy for chronic anal fissure [27] as well as the recognition that IAS division could result in impairment of voluntary squeeze performance [28, 29]. This data should be considered alongside that which showed that, before IAS division for chronic anal fissure in some patients, three-dimensional endoanal magnetic resonance imaging showed a relatively deficient subcutaneous portion of the EAS, leaving the distal anal canal relatively unsupported after internal anal sphincterotomy, thus potentially predicting postoperative functional disturbance [30]. With respect to the importance of the anal cushion as an important mechanism in the maintenance of continence, it has been suggested that sphincteric closure alone is insufficient to maintain continence at rest and that additional mechanisms of hermetic anal occlusion are provided by the columns of Morgagni and the hemorrhoid-bearing anal mucosa and submucosa. Gibbons and colleagues [1] have suggested that there is a linear relationship between the tension of the sphincter and the anal canal diameter, in accordance with the Law of Laplace. The actual principle of importance here is Pascal’s law, which requires that the pressure within a closed system be equivalent at equilibrium; however, in in vivo systems, the wall tension differs in different parts of the canal. The Law of Laplace proposes that, for a defined canal geometry, the tension, pressure, and radius have a defined relationship [31]. The implication here is that the anal canal can be subject to mathematical modeling in the same way as the heart and lungs. This implies that the geometry of the anal canal will provide a variable but predictable tension-pressure relationship, with the closest predictability of the two parameters if the shape is presumed to be spherical. The slope of the graph will be increased by EAS contraction or reduced by IAS relaxation; there are changes in the elastic modulus of the sphincter or the intrinsic diameter of the anal canal when the tension is zero. This effect has been attributed to the anal cushions as a closing mechanism – a view that is not just theoretical in that it shows why reported comparisons of continence disorders after sphincterotomy and hemorrhoidectomy are invalid because the mechanisms of anal closure after these procedures differ.
The Law of Laplace is only valid for cylindrical systems, where the shape of the anal canal determines the circumferential and longitudinal tensions. Total collapse of the anal canal would require a vacuum (somewhat like the lung), and the crenelated margins of the mucosa complicates the geometry being affected by radial traction; fluid within the crenelated folds makes the canal particularly compliant and alters the circumference at rest with relatively little resistance. It is only in states where the diameter is small that fluid covering the mucosal surfaces and luminal surface tension become important. Equally, the elastic modulus of the anal canal will tend to increase in small diameters, or, alternately, the thickness and tension might vary, increasing as the canal occludes and functioning as hydraulic valves at the point of anal closure. Recently, Thekkinkattil and colleagues have shown inherent differences in the measurable anal cushion area in patients presenting with idiopathic fecal incontinence [32], although these data should be viewed with caution because the issues of incontinence after hemorrhoidectomy are complex. It is likely that there are inherent differences in anal closure, but equally there are deep-seated cases of postoperative underlying intersphincteric sepsis (probably underestimated), variations in postoperative rectoanal inhibition, disorders of rectoanal pressure gradients, inadvertent IAS injury (particularly when less invasive operative procedures are performed without separation of the IAS from the submucosa), and variations in preoperative continence and mucosal sensitivity in those cases presenting initially with substantial hemorrhoidal prolapse [33–36].
References
1. Gibbons C, Trowbridge EA, Bannister JJ, Read NW. Role of anal canal cushions in maintaining continence. Lancet. 1986;1:886–8.
2. Schweiger M. Method for determining individual contributions of voluntary and involuntary anal sphincters to resting tone. Dis Colon Rectum. 1979;22:415–6.
3. Allen ML, Zamani S, Dimarino AJ, Sodhi S, Miranda LA, Nusbaum M. Manometric measurement of anal canal resting tone: comparison of a rectosphincteric balloon probe with a water-perfused catheter assembly. Dig Dis Sci. 1998;43:1411–5.
4. Sangwan YP, Solla JA. Internal anal sphincter: advances and insights. Dis Colon Rectum. 1998;41:1297–311.
5. Zbar AP, Jayne DG, Mathur D, Ambrose NS, Guillou PJ. The importance of the internal anal sphincter (IAS) in maintaining continence: anatomical, physiological and pharmacological considerations. Colorectal Dis. 2000;2:193–202.
6. Fritsch H, Brenner E, Lienemann A, Ludwikowski B. Anal sphincter complex: reinterpreted morphology and its clinical relevance. Dis Colon Rectum. 2002;45:188–94.
7. Williams AB, Kamm MA, Bartram CI, Kmiot WA. Gender differences in the longitudinal pressure profile of the anal canal related to anatomical structure as demonstrated on three-dimensional anal sonography. Br J Surg. 2001;87:1675–9.
8. Taylor BM, Beart RW, Phillips SF. Longitudinal and radial variations of pressure in the human anal sphincter. Gastroenterology. 1984;86:693–7.
9. Zbar AP, Aslam M, Hider A, Toomey P, Kmiot WA. Comparison of vector volume manometry and conventional manometry in anorectal dysfunction. Tech Coloproctol. 1998;2:84–90.
10. Goes RN, Simons AJ, Masri L, Beart RW Jr. Gradient of pressure and time between proximal anal canal and high-pressure zone during internal anal sphincter relaxation: its role in the fecal continence mechanism. Dis Colon Rectum. 1995;38:989–96.
11. Morren GL, Beets-Tan RG, Van Engelshoven JM. Anatomy of the anal canal and perianal structures as defined by phased-array magnetic resonance imaging. Br J Surg. 2001;88:1506–12.
12. Telford KJ, Ali AS, Lymer K, Hospker GL, Kiff ES, Hill J. Fatigability of the external anal sphincter in anal incontinence. Dis Colon Rectum. 2004;47:746–52.
13. Bilali S, Pfeifer J. Anorectal manometry: are fatigue rate and fatigue rate index of any clinical importance? Tech Coloproctol. 2005;9:225–8.
14. Shafik AA. A new concept of the anal sphincter mechanism and physiology of defecation. IX. Single loop continence: a new theory of the mechanism of anal continence. Dis Colon Rectum. 1980;23:37–46.
15. Dalley II AF. The riddle of the sphincters. The morphophysiology of the anorectal mechanism reviewed. Am Surg. 1987;53:298–306.
16. Shafik A, Gamal el-Din MA, el-Sibaei O, Abdel Hamid Z, el-Said B. Involuntary action of the external anal sphincter. Manometric and electromyographic studies. Eur Surg Res. 1992;24:188–96.
17. Li D, Guo M. Morphology of the levator ani muscle. Dis Colon Rectum. 2007;50:1–9.
18. Guo M, Li D. Pelvic floor images: anatomy of the levator ani muscle. Dis Colon Rectum. 2007;50:1647–55.
19. Zbar AP, Guo M, Pescatori M. Anorectal morphology and function: analysis of the Shafik legacy. Tech Coloproctol. 2008;12:191–200.
20. Zbar AP, Guo M, Pescatori M. Anorektale morphologie und function: analyse der arbeiten von Shafik. Coloproctology. 2009;31:269–81.
21. Guo M, Gao C, Li D, Guo W, Shafik AA, Zbar A, et al. MRI anatomy of the anal region. Dis Colon Rectum. 2010;53:1542–8.
22. Milligan ETC, Morgan CN. Surgical anatomy of the anal canal with special reference to ano-rectal fistulae. Lancet. 1934;2:1213–7.
23. Gorsch RV. Anorectal fistula: anatomical considerations and treatment. Am J Surg. 1936;32:302–7.
24. Morgan CN, Thompson HR. Surgical anatomy of the anal canal with special reference to the surgical importance of the internal sphincter and conjoint longitudinal muscle. Ann R Coll Surg Engl. 1956;19:88–114.
25. Oh C, Kark AE. Anatomy of the external anal sphincter. Br J Surg. 1972;59:717–23.
26. Thompson JP, Ross AH. Can the external anal sphincter be preserved in the treatment of trans-sphincteric fistula-in-ano? Int J Colorectal Dis. 1989;4:247–50.
27. Casillas S, Hull TL, Zutshi M, Trzcinski R, Bast JF, Xu M. Incontinence after a lateral internal sphincterotomy: are we underestimating it? Dis Colon Rectum. 2005;48:1193–9.
28. Zbar AP, Aslam M, Allgar V. Faecal incontinence after internal sphincterotomy for anal fissure. Tech Coloproctol. 2000;4:25–8.
29. Shafik A, el-Sibai O, Shafik AA. Is myoelectric activity transmittable from one muscle to another: an experimental study. Int J Surg Investig. 2000;2:165–70.
30. Zbar AP, Kmiot WA, Aslam M, Williams A, Hider A, Audisio RA, et al. Use of vector volume manometry and endoanal magnetic resonance imaging in the adult female for assessment of anal sphincter dysfunction. Dis Colon Rectum. 1999;42:928–33.
31. Richeson AW. Laplace’s contribution to pure mathematics. Natl Math Mag. 1942;17:73–8.
32. Thekkinkattil DK, Dunham RJ, O’Herlihy S, Finan PJ, Sagar PM, Burke DA. Measurement of anal cushions in idiopathic faecal incontinence. Br J Surg. 2009;96:680–4.
33. Ho YH, Seow-Choen F, Goh HS. Haemorrhoidectomy and disordered rectal and anal physiology in patients with prolapsed haemorrhoids. Br J Surg. 1995;82:596–8.
34. Zbar AP, Beer-Gabel M, Chiappa AC, Aslam M. Fecal incontinence after minor anorectal surgery. Dis Colon Rectum. 2001;44:1610–2.
35. Zbar AP. Measurement of anal cushions in idiopathic faecal incontinence. Br J Surg. 2009;96:1373–4.
36. Zbar A, Murison R. Transperineal ultrasound in the assessment of haemorrhoids and haemorrhoidectomy: a pilot study. Tech Coloproctol. 2010;14:175–9.
Rectoanal Inhibitory Reflex
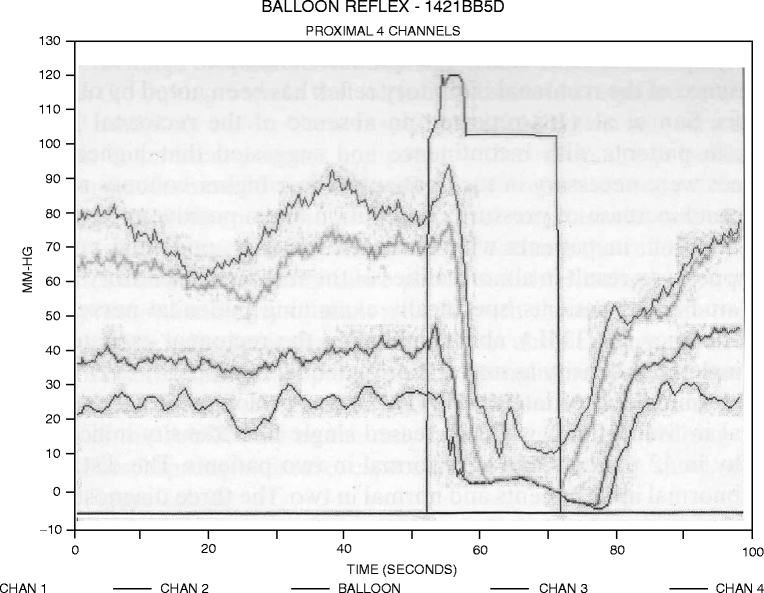
The RAIR is defined as a transient relaxation of the IAS in response to rectal distention with a transient decrease in resting anal pressure and a subsequent return to baseline [13]. The presence of the RAIR is an expression of the integrity of the reflex arch mediated by intramural rectal receptors (afferents) and the sacral plexus (efferents); it has been postulated that the RAIR serves as a sampling reflex, discriminating between flatus and fluid or solid feces [14]. A typical RAIR is shown in Fig. 6.2 .
The RAIR creates a pressure gradient between the rectum and the anal canal that permits defecation. It is absent in Hirchsprung’s disease and absent initially after low anterior resection and ileoanal pouch anal anastomsis. Regeneration may occur after hand-sewn coloanal anastomosis and after low stapled anastomosis [15, 16]. There are a variety of methods for the evaluation of the RAIR, although none have been standardized. The method used by our group is to fill a balloon at the end of the transducer with 60 cm3 or more of water or air in the rectum and measure the response. If the RAIR is present, the distention in the rectum should produce a reflex contraction of the EAS in some cases, followed by relaxation of the IAS, although the technique is operator- and method-dependent. The degree of relaxation varies in the proximal, middle, and distal sphincter, with the greatest degree of relaxation in the proximal IAS. Latency of the RAIR or rectoanal excitatory reflex is measured in seconds from the start of balloon inflation to the onset of the RAIR or rectoanal excitatoty reflex as defined by the computer setting. Many authors have studied the significance of various parameters of the RAIR, including latency, the duration of the reflex, and the amplitude of the reflex in the proximal and distal portions of the sphincter [17, 18].
Greater sphincter relaxation has been observed at each volume of rectal distention in incontinent patients [19, 20]. In this respect, Zbar et al. [21] compared excitatory and inhibitory latencies, maximum excitatory and inhibitory pressures, amplitude and slope of inhibition, slope and time of pressure recovery, and area under the inhibitory curve in patients with fecal incontinence and chronic constipation. They found that the recovery time under the inhibitory curve differed at various levels and among the patient groups, with incontinent patients showing the most rapid recovery. They concluded that continence may rely, in part, on some of these characteristics of rectoanal inhibition and that there may be some parameters that would predict functional results after low anastomosis.
The RAIR is generally absent after low anterior resection, coloanal anastomosis, and ileoanal pouch anal anastomosis; its return over time has been demonstrated after both hand-sewn and stapled anastomoses to correlate with improvement in function and a reduction of nocturnal urgency [15, 16]. It has been shown that the RAIR was present in only 21 % of patients evaluated between 6 and 12 months after low rectal anastomosis, whereas 85 % of patients showed a RAIR 2 years postoperatively [22]. Because the nervous pathways of the RAIR are intramural, they are injured by the dissection of the rectum, and this explains why this reflex is absent postoperatively. Some authors have suggested that neuronal regeneration occurs across the anastomotic site up to 6 months after surgery [23]. Other authors have reported that the RAIR returns as a result of a regeneration of pelvic tension receptors.
Comment: The Rectoanal Inhibitory Reflex
Andrew P. Zbar
The RAIR is defined as a receptive relaxation of anal pressure as a response to rectal distension, an effect that has largely been attributed to the action of the IAS. It was first described by Gowers [1] in 1877 and subsequently confirmed by Denny-Brown and Robertson[2] in 1935. Consensus has defined it as the transient decrease in resting anal pressure by ≥25 % of basal pressure in response to rapid inflation using a rectal balloon, with a subsequent return of the pressure to baseline. The neurophysiology of this reflex is relatively poorly understood, although it is believed to be mediated via the intramural neuronal plexi [3, 4] because it remains intact in patients with spinal cord injury and cauda equine syndrome as well as after full rectal mobilization or presacral nerve blockade. Typically, its absence is used as a diagnostic hallmark of Hirschprung’s disease [5, 6], although this is not always diagnostic in neonates [7, 8], and its return after low anterior resection and anastomosis has been roughly correlated with a return toward “normal” function and loss of fecal urgency, a process that may take up to 2 years after surgery [9, 10]. The elicitation of the RAIR is highly operator-dependent and is intrinsically reliant upon the compliance characteristics of the rectum and the balloon as well as on inherent rectal geometry and the physics of visceral distension. A false-negative reflex may be obtained if the resting anal pressure is low or if the patient has a hyposensitive rectum. Moreover, it is apparent that it is a fatiguable event, so a moratorium needs to be observed between reproducible readings.
In some reports RAIR has been preceded by a transient rise in pressure, defined as a rectoanal excitatory reflex which is particularly noticeable in the distal anal canal. It has been suggested that this correlates with an underlying pudendal neuropathy and its degree [10, 11]. Reproducibility of this reflex is a cardinal component of its elicitation, and we would contend that in some articles that have shown results of a rectoanal excitatoty reflex, excessive volumes have been used to produce a sustainable RAIR, an effect that may stimulate the voluntary sphincter to produce initial increases in the pressure trace. It has been suggested that nitric oxide is the principal chemical mediator of the RAIR via a group of intramural nonadrenergic, noncholinergic neurons [12] and that the mechanoreceptors for RAIR are believed to be located in the mucosa because its elicitation is blocked by the instillation of topical anesthetic gel [13] or by the use of ganglion-blocking agents [14].
There are a variety of methods that can be used to elicit the RAIR, including a range of catheters and balloons and variations between the use of air or water and inflation protocols. Generally, a spiral catheter is used with air inflation based on the first sensory threshold volume to rectal distension; rectoanal excitation and inhibition are confirmed when pressure increases or decreases occur within two standard deviations of the resting pressure. That there are differences in the elicitable RAIR between the proximal, middle, and distal anal canals is evident, and, generally, the greatest degree of relaxation is evident in the proximal anal canal. As part of standard testing, most laboratories tend to define the presence or absence of the reflex, but it is also evident that in reproducible traces there are individual parameters within the different levels of the anal canal that can be measured, including the latency of reflex elicitation, the slope of inhibition, the slope of recovery, the overall recovery time, and the area under the inhibitory curve. Current manometric software provides a ready ability for the determination of these parameters, suggesting a somewhat disease-specific variation in the parametric assessment of the RAIR [15]. In some laboratories, individually recognizable patterns of the RAIR have been shown in incontinent patients. Patients with an EAS defect have no distal excitation but normal proximal inhibition, and those with idiopathic incontinence show distal inhibition with normal proximal inhibition. It has been suggested that, in association with other indicators of poor function (such as a thin or damaged IAS), specific postoperative parametric values may better define those patients about to undergo an ultralow anastomosis who could potentially benefit from construction of a neorectal reservoir. This effect also will have some consequence in children, in whom IAS-preserving techniques have been used [16]. It is also potentially possible that these preoperative parameters could better define those patients with topically resistant chronic anal fissure whose postoperative function would be disadvantaged by sphincter ablation, although such prospective data using this technology is currently not available to guide sphincter-sparing surgery.
References
1. Gowers WR. The automatic action of the sphincter ani. Proc R Soc Lond B Biol Sci. 1877;26:77–84.
2. Denny-Brown D, Robertson EG. An investigation of the nervous control of defaecation. Brain. 1935;58:256–310.
3. Roberts PL. Rectoanal inhibition. In: Wexner SD, Zbar AP, Pescatori M, editors. Complex anorectal disorders: investigation and management. London: Springer; 2005. p. 39–47.
4. Lubowski DZ, Nicholls RJ, Swash M, Jordan MJ. Neural control of internal anal sphincter function. Br J Surg. 1987;74:688–90.
5. Lanfranchi GA, Bazzocchi G, Federici S, Brignola C, Campieri M, Rossi F, et al. Anorectal manometry in the diagnsos of Hirschprung’s disease – comparison with clinical and radiological criteria. Am J Gastroenterol. 1984;79:270–5.
6. De Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschprung;s disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006;42:496–505.
7. Kawahara H, Kubota A, Hasegawa T, Okuyama H, Ueno T, Watanabe T, et al. Anorectal sleeve micromanometry for the diagnosis of Hirschprung’s disease in newborns. J Pediatr Surg. 2007;42:2075–9.
8. Huang Y, Zheng S, Xiao X. Preliminary evaluation of anorectal manometry in diagnosing Hirschsprung’s disease in neonates. Pediatr Surg Int. 2009;25:41–5.
9. Suzuki H, Matsumoto K, Amano S, Fujioka M, Honzumi M. Anorectal pressure and rectal compliance after low anterior resection. Br J Surg. 1980;676:655–7.
10. O’Riordain MG, Molloy RG, Gillen P, Sangwan Y, Coller JA, Barrett RC, et al. Distal rectoanal excitatory reflex: a reliable index of pudendal neuropathy. Dis Colon Rectum. 1995;38:916–20.
11. Sangwan YP, Coller JA, Barrett RC, Murray JJ, Roberts PL, Schoetz DJ Jr. Prospective comparative study of abnormal distal rectoanal excitatory reflex, pudendal nerve terminal motor latency and single fiber density as markers of pudendal neuropathy. Dis Colon Rectum. 1996;39:894–8.
12. O’Kelly TJ, Brading AF, Mortensen NJM. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut. 1993;34:689–93.
13. Meunier P, Mollard P. Control of the internal anal sphincter (manometric study of human subjects). Pflugers Arch. 1977;370:233–9.
14. Kumar D, Phillips SF. Human myenteric plexus: confirmation of unfamiliar structures in adults and neonates. Gastroenterology. 1989;9:1021–8.
15. Zbar A, Ramesh J. Parameters of the rectoanal inhibitory reflex in different anorectal disorders. Dis Colon Rectum. 2003;46:557–8.
16. Lin CL, Chen CC. The rectoanal relaxation reflex and continence in repaired anorectal malformations with and without an internal sphincter saving procedure. J Pediatr Surg. 1996;31:630–3.
Bearing Down
Manometry helps to detect abnormalities during attempted defecation [24]. When a subject attempts to defecate normally, rectal pressure rises. This rise is synchronized with a fall in anal sphincter pressure, in large part due to relaxation of the EAS (bearing down). This maneuver is under voluntary control and is primarily a learned response. The inability to perform this coordinated movement represents the chief pathophysiologic abnormality in patients with dyssynergic defecation. This inability may be due to impaired rectal contraction, paradoxical anal contraction, impaired anal relaxation, or a combination of these mechanisms.
On the basis of these features, Rao et al. [25] recognized three types of dysfunction:
Type 1: The patient can generate an adequate pushing force (a rise in intra-abdominal and intrarectal pressures along with a paradoxical increase in the anal sphincter pressure).
Type 2: The patient is unable to generate an adequate pushing force (no increase in intrarectal pressure) but can exhibit a paradoxical anal contraction.
Type 3: The patient can generate an adequate pushing force (an increase in intrarectal pressure) but has absent or incomplete (<20 %) sphincter relaxation (no decrease in anal sphincter pressure).
These mechanisms create discriminatory anorectal propulsive activity so that during attempted defecation, some subjects may not produce a normal relaxation largely because of the laboratory conditions. In this regard, Rao et al. [25] observed that 80 % of patients with pathologic ambulatory tests showed appropriate sphincter relaxation when manometry was performed at home. When bearing down in the left lateral decubitus, 22 % of healthy subjects exhibited an obstructive pattern of defecation, whereas when sitting on a commode, most (95 %) showed a normal pattern. Hence, if a subject exhibits an obstructive pattern of defecation in the lying position, it is important to repeat the maneuver in a sitting position, preferably with a rectal balloon inflated with air to provide a sensation of rectal fullness.
The occurrence of this pattern alone, however, should not be considered diagnostic of dyssynergic defecation. Differences between men and women when bearing down have been observed. Men have a higher defecation index than women, but this is not statistically significant, suggesting that men are less likely than women to exhibit dyssynergic defecation. Further, older men have a lower defecation index and generally take longer to expel a rectal balloon, which suggests that aging is associated with some impairment of pelvic floor function. When coughing, sneezing, or blowing up a party balloon there is an increase in the intra-abdominal pressure that triggers a reflex rise in the anal sphincter pressure, possibly mediated through a sacral neuronal reflex arc. This reflex response is useful for defining the anatomic level of neuronal injury in patients with incontinence. Because the effort used during coughing is highly variable among subjects, it is both poorly controlled and less reproducible than the effort required to blow up a party balloon.
Residual anal pressure has been defined as the difference between the baseline pressure and the lowest (residual) pressure within the anal canal when the subject is bearing down. Subjects with an obstructive pattern of defecation have a residual anal pressure that is higher than the resting pressure. The percentage of anal relaxation is calculated using the following formula:percentage anal relaxation = anal relaxation pressure/anal resting pressure × 100
To provide an overall index of the changes in the rectal and anal pressures during simulated defecation, a defecation index is calculated, where:defecation index = maximum rectal pressure (strain) ÷ minimal anal residual pressure (strain)
Rectal Sensation
Rectal sensation is the lowest volume of air that evokes a first sensation, a desire to defecate, urgency, or discomfort during intermittent distension. It is measured by filling the balloon in the rectum with increasing amounts of water or air and recording the volume at which the patient first feels the balloon, when they feel a desire to defecate, and at the point of maximal tolerated volume. Poor rectal sensation is seen in patients whose volume when they first feel the balloon and maximal tolerated volume are high. Low rectal sensation often is observed in constipated patients, whereas high sensation is typical of patients with urge incontinence [7].
Comment: Anorectal Mucosal Sensitivity
Andrew P. Zbar
In addition to basic balloon testing, there is associated literature regarding anorectal mucosal sensitivity testing. Although it has been postulated that anorectal sensitivity is important in defecation and in the maintenance of continence, little is known concerning its neural pathways and its laboratory assessment is limited. Clearly it is important in a range of pain-related syndromes including irritable bowel syndrome, proctalgia fugax, and idiopathic anal pain. Anatomically, Duthie and Gairns [1] described the significance of nerve endings in the anorectum, although they had previously been noted independently by Sotolo [2] and Fan [3] in the 1950s. They are most concentrated around the anal transitional zone, where they are represented as Meissner’s touch corpuscles, Krause thermal end-bulbs, friction corpuscles, Golgi-Mazzoni pressure bodies, and Paccinian distension corpuscles, similar to those located in the skin. Superficial mechanoreceptors are located in the mucosa and travel to the presacral ganglia, with deeper layers (including the serosa and mesentery) housing deep receptors that synapse in the lumbar cord via the pelvic splanchnic nerves. Nociceptor pathways travel in both the sympathetic and parasympathetic chains via the inferior and superior hypogastric plexi to L1/L2 because these are abolished during spinal anesthesia and pudendal nerve blockade. Some of these receptors are stimulated by stretch response, whereas others are activated by chemical, mechanical, and thermal stimuli [4].
The assessment of mucosal sensitivity is part of normal compliance and barostat assessment; patients typically are asked to acknowledge first perceived volume, urge volume, and maximal tolerated volume to balloon distension. These crude assessments cannot provide definitive information regarding rectal capacity, compliance, or rectal wall stiffness [5]. Clearly, the sensation of contents in the rectum is different than that of rectal filling, although they reflect elastic receptive rectal properties without specifically saying anything about mucosal sensibility. These differences will account for differences in reported normal values, with first sensation volumes of 40–90 mL, urge volumes of 140–160 mL, and maximal tolerated volumes of between 200 and 300 mL for different recorded intra-anal pressures [6]. Generally, patients with irritable bowel syndrome are hypersensitive to rectal distension [7], an effect that is evident in both diarrhea-predominant and constipation-predominant irritable bowel syndrome [8] but not in inflammatory proctocolitis [9]. Conversely, in simplistic terms, visceral sensitivity is dulled in long-standing severe idiopathic constipation, particularly that with demonstrated slow transit [10, 11]. The studies of rectal sensitivity in incontinence are mixed, and this is reflective of the variability of compliance measurement in this eclectic group of patients.
However, it is more common for disparities in rectal sensitivity to occur at low recorded compliance values, reflective of a reduced rectal capacity or, in the case of rectal prolapse repair, of the return of the mucosa to a more normal position [12]. The definitive assessment of anal sensitivity is difficult. Traditionally, Duthie and Gairns [1] used hairs, pinpricks, and metal plates of varying temperatures to assess anal sensation, techniques that were improved slightly by Rogers [13] and Miller and colleagues [14, 15] independently by the infusion of fluids of varying temperature. Roe et al. [16] were the first to use intra-anal, electrodes providing a square wave current of variable intensity originally designed by Siegel [17], although this technique of “mucosal electrosensitivity” (MES) may work through a number of different afferent pathways [18]. This system uses a specially designed 10-Fr catheter mounted with two platinum wire electrodes 1 cm apart. The square stimulus constant current of 100 ms pulsed at 5 pulses/s is serially increased from 1 to 20 mA until the patient recognizes the stimulus (usually a tingling sensation), with normal values recorded as between 2 and 7 mA, depending on patient age; older patients have an overall lower sensitivity [19]. In general, although there is considerable overlap, incontinent patients have a high MES, particularly if there is an EAS defect. The recorded MES values tend to increase after vaginal delivery, although this is a recoverable event [20]. Equally, obstructed defecation is associated with a higher MES [21], whereas those with chronic anal fissure have lower MES values. Patients with hemorrhoids have a higher MES, which often rises after hemorrhoidectomy. In rectopexy for prolapse, the results are mixed, often with no effect on MES. Excision of the anal transitional zone in coloanal anastomosis reduces the recorded MES, which has been one of the reasons given for low stapled pouch anal anastomosis without mucosal proctectomy preserving postoperative function and sensation [22–24]. This has been associated with reduced overall MES values after ileal pouch anal anastomosis with mucosal proctectomy, with which there is significant reported soiling [24], although this is dependent upon age [25].
References
1. Duthie HL, Gairns FW. Sensory nerve endings and sensation in the anal region of man. Br J Surg. 1960;67:585–9.
2. Sotolo JR. Nerve endings of the walls of the descendent colon and rectum. Z Zellforsch. 1954;41:S101–11.
3. Fan WW. Histological studies of sensory nerves in the sigmoid and rectum. Arch Fur Jap Chir. 1955;24:567–74.
4. Rogers J, Hayward M, Henry M, Misiewicz JJ. Temperature gradient between the rectum and anal canal: evidence against the role of temperature sensation as a sensory modality in the anal canal of normal subjects. Br J Surg. 1988;75:1083–5.
5. Broens PMA, Penninckx FM, Lestar B, Kerremans R. The trigger for rectal filling sensation. Int J Colorect Dis. 1994;9:1–4.
6. Felt-Bersma RJF. Anorectal sensitivity. In: Wexner SD, Zbar AP, Pescatori M, editors. Complex anorectal disorders: investigation and management. London: Springer; 2005. p. 137–52.
7. Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable bowel syndrome. Gut. 1973;14:125–32.
8. Prior A, Maxton DG, Whorwell PJ. Anorectal manometry in irritable bowel syndrome: differences between diarrhoea and constipation predominant subjects. Gut. 1990;31:458–62.
9. Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnsons TD, Bernstein CN, et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2002;47:497–505.
10. Mertz H, Naliboff B, Mayer EA. Physiology of refractory constipation. Am J Gastroenterol. 1999;94:609–15.
11. Penning C, Steens J, van der Schaar PJ, Kuyvenhoven J, Delamarre JB, Lamers CB, et al. Motor and sensory function of the rectum in different subtypes of constipation. Scand J Gastroenterol. 2001;36:32–8.
12. Siproudhis L, Bellissami E, Juguet F, Mendier M-H, Allain H, Bretagne J-F, et al. Rectal adaptation to distension in patients with overt rectal prolapse. Br J Surg. 1998;85:1527–32.
13. Rogers J. Rectal and anal sensation. In: Swash M, Henry MM, editors. Coloproctology and the pelvic floor. Oxford, UK: Butterworth Heinemann; 1992. p. 54–60.
14. Miller R, Bartolo DCC, Cervero F, Mortensen NJM. Anorectal temperature sensation: a comparison of normal and incontinent patients. Br J Surg. 1987;74:511–5.
15. Miller R. The measurement of anorectal sensation. In: Kumar D, Waldron D, Williams NS, editors. Clinical measurement in coloproctology. London: Springer-Verlag; 1991. p. 60–6.
16. Roe AM, Bartolo DCC, Mortensen NJM. New method for the assessment of anal sensation in various anorectal disorders. Br J Surg. 1986;73:310–2.
17. Siegel H. Cutaneous sensory threshold stimulation with high frequency square wave current. J Invest Dermatol. 1951;18:441–5.
18. Vierck CJ, Greenspan JD, Ritz LA, Yeomans DC. The spinal pathways contributing to the ascending conduction and descending modulations of pain sensation and reactions. In: Yaksh TL, editor. Spinal Afferent Processing. New York: Plenum Press; 1986. p. 275–329.
19. Felt-Bersma RJF, Poen AC, Cuesta MA, Meuwissen SGM. Anal sensitivity test: what does it measure and do we need it? Dis Colon Rectum. 1997;40:811–6.
20. Cornes H, Bartolo DC, Stirrat GM. Changes in anal canal sensation after childbirth. Br J Surg. 1991;78:74–7.
21. Solana A. Roig JV, Villoslada C, Hinojosa J, Lledo S. Anorectal sensitivity in patients with obstructed defecation. Int J Colorect Dis. 1996;11:65–70.
22. Holdsworth PJ, Johnston D. Anal sensation after restorative proctocolectomy for ulcerative colitis. Br J Surg. 1988;75:993–6.
23. Komatsu J, Oya M, Ishikawa H. Quantitative assessment of anal canal sensation in patients undergoing low anterior resection for rectal cancer. Surg Today. 1995;25:867–73.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree