Nutrient
Etiology
Deficiency conditions
Assessment/Monitoring
Treatment
Thiamine (vitamin B1)
Precipitated after 1–3 months of intractable persistent vomiting
Wernicke’s syndrome (double vision, nystagmus)
Diagnosis typically made by clinical presentation
Acute deficiency
Acute polyneuropathy
Laboratory confirmation:
Thiamin 100 mg IV or IM × 7–14 days, then 10 mg/day orally until patient fully recovers
Korsakoff encephalopathy (mental confusion)
Low basal erythrocyte transketolase activity (ETKA), enhanced response after TPP addition
Prophylaxis
Dry beriberi (paresthesia)
Increased T2 signal on MRI of brain in thalamus and midbrain
1.5–1.8 mg po qd
Wet beriberi (high output CHF)
Iron
Diminished consumption of iron-rich foods (meats) due to intolerance
Iron deficiency is asymptomatic
Serum ferritin (reflects size of the storage of iron compartments (normal >12 μg/L)
All patients should take a multivitamin/mineral supplement containing 28–40 mg iron/day
Achlorhydria—acid needed to reduce ferric to ferrous state
Iron deficiency Anemia (IDA)—fatigue, poor exercise tolerance, pale conjunctiva, spoon nails
Iron supplementation with ferrous iron 40-65 mg orally TID (+vitamin C)
Bypassed site for absorption
Pica (pagophagia)
Increased total iron-binding capacity (TIBC), reduced transferrin saturation, microcytosis, microcytic anemia
Parenteral iron with iron dextran, sucrose or gluconate, ferumoxytol
Iron losses in menstruating women
Vitamin B12
Decreased acid and pepsin digestion of protein-bound B12 from foods
Asymptomatic until development of anemia
Serum vitamin B12
Vitamin B12 350–1,000 μg orally or sublingually or
Achlorhydria—acid needed to convert pepsinogen to pepsin
With advanced deficiency, development of polyneuropathy, paresthesias, and permanent neural impairment
<200 pg/mL
Nasal spray (Nascobal) 500 μg q weekly or
Inadequate release of intrinsic factor (IF)
Macrocytic anemia, hypersegmented polymorphonuclear leukocytes
Intramuscular injection
Incomplete release of B12 from R binders
Elevated serum homocysteine and methylmalonic acid (MMA) levels
100 μg monthly
Calcium
Tetany
Low serum calcium
Prophylaxis
Osteoporosis
Elevated parathyroid hormone (PTH) level
Calcium citrate 1,200–2,000 mg q daily
Calcium and Vitamin D
Reduced intake of calcium and vitamin D-containing foods
Asymptomatic until development of osteoporosis or
Increased alkaline phosphatase
All patients should take 1,200–2,000 mg calcium and ~3,000 IU vitamin D daily
Malabsorption/decreased absorption due to bypassed intestine and poor mixing of pancreatic and biliary secretions
Osteomalacia. May present as a bone fracture
Serum 25(OH)D level <30 ng/mL
If deficiency, 50,000 IU vitamin D orally three times weekly; repeat 25(OH)D and PTH in several months
Dark skin pigmentation, poor sun exposure, and obesity
Increased parathyroid hormone (PTH)—secondary hyperparathyroidism
Micronutrient Deficiency
Thiamine
Thiamine (vitamin B1) is a coenzyme for the essential enzymes transketolase, pyruvate dehydrogenase, and pyruvate carboxylase, in the early stages of the tricarboxylic acid cycle and in the pentose phosphate pathway [25]. Thiamine is mainly absorbed in the jejunum by both active and passive diffusion. Since the biological half-life of the vitamin is rather short (in the range of 9–18 days) and only a small percentage of a high dose is absorbed [26], patients are at risk of developing deficiency syndromes after bariatric surgery. Over the past 3 decades, numerous case reports of thiamine deficiency have been reported following both restrictive and restrictive-malabsorptive surgeries [27–44]. An acute deficiency of thiamine associated with rapidly progressing clinical symptoms appears to most commonly result from a combination of restricted food intake and persistent intractable vomiting. Symptoms commonly occur 1–3 months postoperatively although may occur later. The clinical presentation varies but three conditions have been reported. Classical Wernicke’s encephalopathy is the most common presentation and consists of double vision, nystagmus, ataxia, and a global confusion manifested by apathy, impaired awareness of the immediate situation, disorientation, inattention, and an inability to concentrate. Dry beriberi presents as bilateral, symmetric, lower extremity paresthesia, while wet beriberi manifests as high output congestive heart failure, edema, and metabolic acidosis.
Several recent reviews of neurologic complications following bariatric surgery have been published [45–50]. These authors describe a constellation of symptoms including mono- and polyneuropathy with weakness and/or paresthesias, burning feet syndrome, and hyporeflexia. Chang et al. [51] coined the acronym APGARS (Acute post-gastric reduction surgery neuropathy) to describe conditions with features of weakness, hyporeflexia, and vomiting. Since all symptoms did not improve with thiamine treatment, the authors suggest that additional nutritional deficiencies may be involved in the etiology of this syndrome.
Thiamine status is best assessed by determining erythrocyte transketolase activity. Magnetic resonance imaging (MRI) is useful in confirming the diagnosis of acute Wernicke’s encephalopathy with a sensitivity of 53 % and specificity of 93 % [52]. With this test, increased T2 signal of paraventricular regions of the thalamus and increased T2 signal of periaqueductal regions of the midbrain are seen. However, treatment should not be delayed if a thiamine deficiency syndrome is suspected. Treatment with thiamine 100 mg IV every 8 h for 7–14 days followed by 50–100 mg po daily is recommended for these syndromes until the patient fully recovers. To avoid deficiency, patients should be routinely discharged from the hospital receiving a chewable multiple vitamin-mineral supplement that contains between 1.5 and 1.8 mg thiamine.
Vitamin B12
Vitamin B12 (cobalamin) is a cofactor in the biosynthesis of succinyl-coenzyme A and methionine and is important for the functioning of hemopoetic and neural cells [25]. Vitamin B12 absorption requires a complex sequence of orchestrated metabolic steps within the gastrointestinal tract (Fig. 18.1). In the stomach, food-bound B12 is first dissociated from animal proteins by acid and peptic hydrolysis to liberate free vitamin B12. Once released, the vitamin is avidly bound to R proteins, which are glycoproteins secreted by the salivary glands and the gastric mucosa. In the intestine, pancreatic proteases then degrade R proteins and permit vitamin B12 to associate with intrinsic factor (IF), a glycoprotein that the parietal cells of the stomach secrete after being stimulated by food. The resulting IF-vitamin B12 complex is then bound to specific receptors in the distal ileum, where absorption occurs [26].
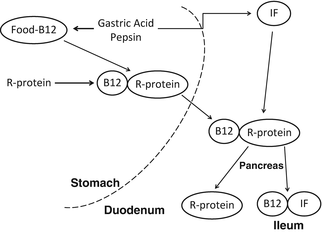

Fig. 18.1
Vitamin B12 absorption. See text for individual metabolic steps in absorptive process
The restrictive-malabsorptive procedures disrupt several of these key steps. Vitamin B12 deficiency may occur due to decreased acid and pepsin digestion of protein-bound cobalamins from food, incomplete release of vitamin B12 from R proteins, and decreased availability of IF to form IF-vitamin B12 complexes. Because the parietal cells which secrete acid and IF, and chief cells which secrete pepsinogen, are located primarily in the fundus and body of the stomach, the LGS and RYGB procedures essentially excludes food from the normal gastric digestive process. Acid secretion has been demonstrated to be virtually absent in the small pouch constructed from the gastric cardia [53, 54]. Consequently, cobalamins are not liberated from protein and are not available for intestinal absorption. In all three restrictive-malabsorptive procedures, pancreatic secretions are diverted distally to mix with nutrients in a shortened common channel, thus affecting the vitamin’s binding to IF and subsequent attachment to ileal IF-vitamin B12 receptors.
Although B12 deficiency is predictable, onset of signs and symptoms are typically delayed for months to years due to prolonged hepatic storage of the vitamin. When they do occur, clinical effects of deficiency are similar to those of pernicious anemia—hematological and neurological. Hypersegmented polymorphonuclear leukocytes and macrocytic erythrocytes can be seen on peripheral blood smear along with a macrocytic anemia. Neurological manifestations include sensory disturbances in the lower extremities (tingling and numbness); motor disturbances including abnormalities in gait; and cognitive changes ranging from loss of concentration to memory loss and disorientation [26].
Vitamin B12 status is most commonly and easily assessed by serum or plasma vitamin levels. The concentration of B12 in the serum or plasma reflects both the B12 intake and stores. The lower limit is considered to be approximately 120–180 pmol/L (170–250 pg/mL). However, a more sensitive biochemical indicator of deficiency is elevation of serum homocysteine and methylmalonic acid (MMA), levels which rise when the supply of B12 is low and virtually confirms the diagnosis.
All patients who undergo restrictive-malabsorptive procedures should receive prophylactic vitamin B12 supplementation to prevent deficiency. In contrast to the disruption of food-bound B12 absorption, crystalline vitamin B12 (the form found in vitamin supplements) can be absorbed in the surgical patient since approximately 1 % of orally administered crystalline cobalamin is absorbed by passive diffusion [55, 56]. An oral dose of at least 200 times the recommended dietary allowance (RDA) was shown to normalize mild vitamin B12 deficiency in older people assessed by reduction in plasma MMA concentration [57]. Oral treatment has also been effective in patients with pernicious anemia [58]. As a practical matter, patients should receive at least 500 μg B12 daily as a dietary supplement delivered orally as a tablet or liquid or sublingually; as a once weekly nasal spray 500 μg cyanocobalamin gel (Nascobal®), or by intramuscular injection (100 μg monthly to 3,000 μg every 6 months). The route of delivery is based on patient preference and monitoring of vitamin B12 status.
Folate
Folate deficiency occurs with lower frequency than vitamin B12 deficiency; however, it should be considered when evaluating a patient who develops anemia. Folate is a cofactor in the biosynthesis of methionine, thymidine, and purine nucleotides, and for the synthesis of the coenzyme tetrahydrofolate [25]. Folate is absorbed primarily from the proximal third of the small intestine after food folate polyglutamates are hydrolyzed to monoglutamates by intestinal brush border conjugases. Folate deficiency presents with many features similar to vitamin B12 deficiency, including hypersegmentation of the neutrophils, increased mean cell volume (MCV), and macrocytic anemia. Inadequate folate intake first leads to a decrease in serum folate concentration, then a decrease in erythrocyte folate concentration, a rise in homocysteine concentration, followed by clinical hematological changes as mentioned above [26]. A serum folate concentration of less than 7 nmol/L (3 ng/mL) indicates negative folate balance. All patients undergoing a restrictive-malabsorptive bariatric operation should receive supplemental doses of folate to prevent deficiency. Supplements of folic acid are nearly 100 % bioavailable. Typically, the amount of folate present in a general (400 μg) or prenatal multivitamin supplement (800–1,000 μg) is adequate to prevent deficiency.
Iron
Patients who undergo restrictive-malabsorptive procedures are at particular risk for developing iron deficiency and iron deficiency anemia (IDA) due to reduced iron absorption, decreased iron intake, and for menstruating women, increased iron losses. Surgical bypass of the duodenum and proximal jejunum decreases total iron uptake because the majority of iron absorption occurs across the apical and basolateral membrane of duodenal enterocytes [59]. Furthermore, acid secretion is nearly absent in the small gastric pouch [53, 54] or remnant gastric sleeve due to the paucity of parietal cells. Hypoacidity exacerbates iron deficiency because both heme (found only in animal products) and nonheme (found in cereals and green leafy vegetables) iron depend upon the acidic environment of the stomach for efficient absorption [60]. Specifically, nonheme iron requires an acidic pH to reduce it from the ferric (Fe3+) to the ferrous (Fe2+) state, before it can be transported across the brush border membrane by divalent metal ion transporter 1 (DMT 1) in the alkaline duodenum. Although heme iron is more soluble and readily absorbed than nonheme iron, it must be released from its protein structure by the acid and proteases present in gastric juice before absorption can occur [61]. If iron is required by the body, it will cross the basolateral membrane through iron export protein ferroportin and enter the circulation in which is binds to plasma transferrin [62]. Iron absorption from the diet or from supplementation has been shown to decrease significantly after 6 months following RYGB to 33 % and 40 % of their initial values, respectively [63].
In addition to decreased iron absorption, bariatric surgical patients typically consume less heme iron due to an intolerance of meat products (which is full of hemoglobin and myoglobin) [64]. Women with menorrhagia are particularly prone to develop iron deficiency and IDA due to excessive menstrual blood loss. Menstrual iron losses range from 1.5 to 2.1 mg/day, bringing the RDA for females between 19 and 50 years old to 18 mg/day compared to 8 mg/day for males [65]. Due to the combination of these factors, IDA occurs postoperatively in 33–50 % of patients, with a higher incidence in menstruating women [66–68].
Iron deficiency may also be exacerbated in these patients as a result of a nutrient–nutrient inhibitory absorptive interaction between iron and calcium, another mineral that is routinely supplemented during the postoperative period. Most [69–74] but not all [75, 76] studies show that nonheme- and heme-iron absorption is inhibited up to 50–60 % when consumed in the presence of calcium supplements or with dairy products. Calcium at doses of 300–600 mg has a direct dose-related inhibiting effect on iron absorption. This has been seen with calcium carbonate, calcium citrate, and calcium phosphate. Studies by Hallberg et al. [70, 72] suggest that the inhibitory effect is situated within the intestinal mucosal cells. These observations are particularly important for bariatric surgical patients who are routinely prescribed calcium supplements and advised to consume dairy foods high in calcium, such as milk, cheese, and yogurt. In these patients, it appears prudent to recommend that iron and calcium supplementation be separated by several hours to avoid inhibitory interaction.
Early functional symptoms of iron deficiency include fatigue, poor exercise tolerance, and decreased work performance [77]. Signs on physical examination include pale conjunctiva and spoon nails. Serum ferritin is the most sensitive indicator of iron status (normal values usually fall in the range of 20–300 μg/L) and is recommended for diagnosing early iron deficiency [78]. The concentration of serum ferritin reflects the size of the storage iron compartment, with each μg/L representing 8–10 mg of storage iron [60]. However, caution is needed in interpreting ferritin concentration levels in the presence of acute and chronic inflammation since ferritin is also an acute phase reactant. Thus, serum ferritin concentrations may fall within normal range in individuals who have diminished iron stores. The concentration of liver-derived peptide hepcidin reflects body iron requirements and may be useful in the future as a biomarker for monitoring iron status [62]. Hepcidin regulates iron homeostasis by regulating ferroportin-mediated release of iron from enterocytes and macrophages. After the iron storage pool is depleted, there is an increase in total iron-binding capacity (TIBC), decreased serum transferrin saturation (serum iron concentration divided by TIBC × 100), followed by microcytosis (reduced mean corpuscular volume or MCV), hypochromia (reduced mean corpuscular hemoglobin concentration or MCHC), and anemia.
An unusual and fascinating symptom that is particularly associated with IDA is ice eating, or pagophagia, one of the most commonly reported forms of pica. Pica has been previously reported to occur with IDA of pregnancy [79, 80], gastrointestinal blood loss [81], and sickle cell disease [82]. Our group previously reported the first five cases of pagophagia associated with RYGB surgery [83, 84]. All patients were women between 34 and 45 years old with menorrhagia. Onset of pica symptoms ranged from 1 to 23 months post-surgery. Three of the patients described symptoms suggestive of pica when they were children and one during a previous pregnancy.
In order to prevent iron deficiency, all patients undergoing restrictive-malabsorptive surgeries should be prescribed a daily multivitamin–mineral supplement containing elemental iron. Supplementation with one prenatal vitamin and mineral tablet, which typically contains 28–40 mg elemental iron, is often sufficient. High-risk individuals, for example, those who have preoperative iron deficiency or excessive blood loss or those who develop iron deficiency or any degree of anemia, require additional supplementation with an iron salt preparation. Only ferrous iron formulations should be used such as ferrous sulfate, gluconate, or fumarate, since they are more readily absorbed [85]. However, it is important to note that a tablet of the sulfate salt contains twice the amount of elemental iron (65 mg) as a tablet of the other two salts. Therefore, twice as many ferrous gluconate or ferrous fumarate tablets are required to provide the amount of elemental iron in ferrous sulfate tablets [86]. Typical dosing of iron therapy is 150–200 mg/day po given in 2–3 divided doses for several months or until the serum ferritin reaches 50 μg/L. Patients who are responsive to treatment should develop reticulocytosis followed by an increase in hemoglobin. Co-administration with ascorbic acid (vitamin C), the best known reducing agent, is recommended to increase iron absorption [87]. In the presence of ascorbic acid, ferrous iron forms a soluble iron-ascorbic acid complex. Patients who were anemic may require long-term daily supplementation in addition to their other nutritional supplements. In patients with profound iron deficits and severe anemia unresponsive to oral iron supplementation, intravenous administration of iron dextran (InFed), ferric gluconate (Ferrlecit), ferric sucrose (Venofer), or ferumoxytol (Feraheme) will be required.
Calcium and Vitamin D
Calcium and vitamin D are considered together since deficiency of both nutrients may result in metabolic bone disease and their metabolism is interrelated. A negative calcium balance may result from limited intake of calcium- and vitamin D-containing dairy products, reduced fractional intestinal absorption due to surgical bypass of the absorptive sites, and vitamin D deficiency. The latter factor is important since calcium is absorbed by an active transport process dependent on the action of 1,25-dihydroxyvitamin D (1,25(OH)2D) which binds with high affinity to the vitamin D receptor (VDR) to enhance calcium absorption primarily in the duodenum and jejunum [88] although most of the absorption occurs in the lower segment of the small intestine, the ileum [89]. Calcium is also absorbed by passive diffusion across the intestinal mucosa which becomes important at high calcium intakes such as supplemental calcium [90]. In a prospective study of women who underwent RYGB, fractional radiolabeled calcium absorption in milk was reduced 33 % 6 months after surgery [91].
Vitamin D deficiency may occur for the same reasons listed above for calcium deficiency, that is, reduced intake of vitamin D fortified dairy products and malabsorption of vitamin D due to mismixing of pancreatic and biliary secretions in the distal small intestine. Since vitamin D is fat soluble, it must be incorporated into the intestinal micelle along with bile salts for absorption. However, the major source of vitamin D for most people comes from casual exposure to sunlight. Unlike any other vitamin, vitamin D3, or cholecalciferol is photosynthesized by the skin by UVB irradiation, converting 7-dehydrocholesterol (provitamin D) to previtamin D3 in the plasma membrane of skin keratinocytes. Once formed in the skin, previtamin D3 is rapidly converted to vitamin D3 [90]. In the liver, vitamin D undergoes hydroxylation at the 25-carbon position to form 25-hydroxy vitamin D (25(OH)D) and subsequently transported to the kidney for additional hydroxylation at the 1-carbon position to form 1,25(OH)2D, the biological active form of the vitamin. Several factors will impede the initial photosynthetic process including living at northern latitudes, wearing sunscreen lotion, limited sun exposure, dark skin pigmentation [92], aging, and obesity itself [93–96]. Melanin skin pigmentation is an effective natural sunscreen that greatly reduces UVB-mediated cutaneous synthesis of vitamin D3. Thus dark-skinned individuals need longer UVB exposure to generate the same 25(OH)D stores compared with fair-skinned individuals. Several studies have demonstrated an inverse correlation between vitamin D concentrations and BMI or body fat percentage, suggesting decreased bioavailability of skin-derived vitamin D in obese individuals [97]. Thus, severely obese individuals are predisposed to vitamin D insufficiency or deficiency prior to undergoing bariatric surgery.
Clinical deficiency of calcium or vitamin D due to bariatric surgery cannot be detected on a routine chemistry panel, although an elevated alkaline phosphatase level and low calcium or phosphorus level may be seen. Symptoms of vitamin D deficiency are commonly nonspecific such fatigue or easy tiring, muscular weakness predominantly of the proximal limb muscles, and chronic musculoskeletal pain [98]. Unless specifically monitored, the first indication of deficiency is likely to be a vertebral or wrist fracture secondary to development of osteoporosis or osteomalacia. Physiologically, chronic calcium deficiency causes the circulating ionized calcium concentration to decline, which triggers an increase in parathyroid hormone (PTH) synthesis and release. In turn, PTH acts on three organs to restore the circulating calcium concentration to normal. At the kidney, PTH promotes the reabsorption of calcium in the distal tubule. PTH affects the intestine indirectly by stimulating the production of 1,25(OH)2D. PTH also induces bone resorption, thereby releasing calcium into the blood [88]. Chronic vitamin D deficiency can result in secondary hyperparathyroidism, increased bone turnover, enhanced bone loss, and increased risk of fragility fracture [99]. Secondary hyperparathyroidism is diagnosed by an elevated PTH level in the setting of low or normal serum calcium [92]. Therefore, detection of subclinical calcium and/or vitamin D deficiency requires measurement of several nutrients, hormone levels, and biochemical markers of bone turnover that are not routinely assessed.
Serum 25(OH) vitamin D is the best indicator for determining adequacy of vitamin D intake since it represents the combination of cutaneous production of vitamin D and the oral ingestion of both vitamin D2 (ergocalciferol or plant-based vitamin D) and vitamin D3. 25(OH)D is not only the transport form of the vitamin D but a direct measure of stores [100]. In the current literature, severe vitamin D deficiency is identified as a 25(OH)D level of less than 5–8 ng/mL (12.5–20 nmol/mL) and mild deficiency or insufficiency as a serum level less than 20 ng/mL (50 nmol/mL) [101]. However, there is debate about the exact cutoff values defining ‘deficiency’ and ‘insufficiency’ since these are static rather than functional definitions. When elevated PTH levels (secondary hyperparathyroidism) are used as a functional indicator of vitamin D deficiency, circulating levels of 25(OH)D of at least 30 ng/mL appear optimal [102, 103]. Biochemical monitoring of bone turnover includes measurement of bone formation markers—serum osteocalcin and bone-specific alkaline phosphatase, and the bone resorption marker—serum and urine peptide-bound N-telopeptide crosslinks of type 1 collagen (NTX) [104]. Assessment of bone mineral density (BMD) and bone mineral content (BMC) by dual energy X-ray absorptiometry (DXA) remains the gold standard for the diagnosis of osteoporosis [105].
Abnormalities in vitamin D and bone metabolism among patients undergoing restrictive-malabsorptive bariatric operations have been reported in numerous case series, case reports, and recent reviews [106–118]. Although the studies are primarily observational, contain few patients, and uncontrolled for diet and vitamin mineral supplementation, most studies document the occurrence of hypovitaminosis D and elevated PTH over the first 1–3 postoperative years, with a prevalence ranging from 30 [98] to 80 % [119]. Many of the studies also show a corresponding elevation in alkaline phosphatase levels and biochemical markers of bone turnover. There are multiple factors involved in postsurgical bone mass loss that includes nutritional deficiencies, rapid weight loss, and possibly changes in adipokines and gut-derived appetite regulatory hormones [118]. In a prospective study of 73 patients who underwent either a restrictive or restrictive-malabsorptive bariatric surgical procedure, both urinary NTX and osteocalcin increased significantly by 3 months after surgery and remained significantly elevated through 18 months [120]. Median serum PTH levels at 9 and 12 months were higher in patients who underwent a restrictive-malabsorptive procedure compared to those who underwent LAGB. PTH levels were associated with increased serum 1,25(OH)2D.
It is important to prospectively monitor patients preoperatively and postoperatively since many obese patients present to surgical centers with underlying vitamin and mineral deficiencies, including D deficiency and some with secondary hyperparathyroidism [121–125]. Several cases of severe secondary hyperparathyroidism with osteomalacia have been reported to occur from 9 to 17 years post-surgery [112, 126]. However, since weight reduction itself is associated with reduced BMD and BMC [127, 128], it is important to distinguish between the weight loss and malabsorptive effects of bariatric surgery. Pugnale et al. [129] showed that BMD of the cortical bone decreased significantly among 31 women who underwent a restrictive banding procedure without evidence of secondary hyperparathyroidism. In a 1-year prospective study among 23 obese men and women who underwent RYGB, there was a significant decrease in BMD at the femoral neck and total hip by 9.2 % and 8 %, respectively, associated with a significant increase in urinary NTX and serum osteocalcin [130]. Similarly, Guney et al. demonstrated that weight reduction causes bone loss among both diet treated patients and those who underwent a restrictive vertical banded gastroplasty without a significant change in PTH levels [131]. In another study, six obese control patients were compared to four patients who underwent an RYGB and nine patients who received gastric banding [111]. The RYGB operation resulted in significant net loss of bone mass in comparison to the banding and obese control group. In the study by El-Kadre et al. [109], 10 % of patient had elevated PTH levels preoperatively, whereas the prevalence was 22 % and 25 % in the series by Johnson et al. [114] and Hamoui et al. [110], respectively.
Inclusion of calcium- and vitamin D-containing dairy products in the postoperative diet is important. One serving of milk contains approximately 300 mg calcium. However, many patients will avoid or limit dairy foods due to lactose intolerance or lack of an acquired taste. Choosing lactaid milk or adding lactase to dairy products will address the former problem. To avoid deficiency and supplement the diet, all patients should receive calcium supplements of at least 1,200–2,000 mg/day in divided doses, depending on the adequacy of dietary calcium. Postmenopausal, lactating or pregnant women may require higher ranges due to increased needs. Calcium citrate + vitamin D is the preferred preparation because it is more soluble than calcium carbonate in the absence of gastric acid production [132]. The optimal dose for vitamin D is more controversial since some studies have shown continued deficiency despite high dose supplementation [116, 120, 121]. General guidelines recommend 3,000–5,000 IU per day. Since multivitamin–mineral tablet typically contains 400 IU and calcium + Vitamin D tablets typically contain 500 IU, many patients will require additional vitamin D.
If vitamin D deficiency is detected, measurement of PTH should be obtained to provide a functional assessment. Treatment may involve recommending higher supplemental doses of calcium and vitamin D and reassessing in about 3 months. In patients with severe vitamin D deficiency, initial repletion of stores should be treated with vitamin D 50,000 IU 1–3 times weekly for 8 weeks, then checking 25(OH)D levels [111]. Monitoring of the alkaline phosphatase level and serum and urinary calcium should also be performed.
Other Deficiencies
Micronutrient deficiencies of vitamin A and copper have been reported following bariatric surgery, although less often that the aforementioned nutrients. They will briefly be reviewed.
Vitamin A
Vitamin A, whether ingested as preformed vitamin A (retinyl esters) or as provitamin A carotenoids, requires processing in the intestine to release the nutrients in an absorbable form [25]. Since vitamin A is a fat-soluble vitamin, any condition that interferes with emulsification is likely to reduce intestinal absorption of retinol. Thus vitamin A dietary compounds are more likely to be malabsorbed with the BPD and BPDDS procedures which limit the exposure of food with biliopancreatic digestive secretions within a shortened common channel. Subsequently, deficiency of vitamin A has been more frequently reported among patients who have undergone a BPD or BPDSS procedure compared to RYGB [16], although deficiency has been reported following RYGB as well [133–135]. Slater et al. [136




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






