Marker
Function
Comments
Classically activated macrophage (M1 macrophage)
IL-6
Promotes inflammatory response
Induced by IFN-γ
IL-12
Induces Th1 development
Induced by IFN-γ
iNOS
Produces NO to kill microorganisms
Induced by IFN-γ
TNF-α
Promotes inflammatory response
Induced by IFN-γ
Alternatively activated and regulatory macrophage (M2 macrophage)
Arg1
Counteracts iNOS
Induced by STAT3/6 pathways
IL-10
Anti-inflammatory cytokine
Induced by TLRs-signaling
PD-L2
Inhibits T cell activation
Induced by STAT6 pathway
RELMα
Promotes deposition of ECM
Induced by IL-4 and IL-13
TGFβ
Anti-inflammatory cytokine
Induced by IL-13
YM1
Binds to ECM
Induced by IL-4
It is generally believed that macrophages represent a spectrum of activated phenotypes rather than discrete stable subpopulations. Indeed, many studies have reported flexibility in their programming, with macrophages switching from one functional phenotype to another [16, 23, 44]. In addition, although the M1/M2 classification is a useful heuristic that may reflect extreme states, this classification is unable to represent diverse forms of macrophage activation in vivo, which is induced by the variable microenviromental signals of the local milieu. Indeed, transcriptional profiling of resident macrophages by the Immunological Genome Project (http://www.immgen.org/) represents that these populations have high transcriptional diversity with minimal overlap, suggesting that there are several unique classes of macrophages [13]. Thus, macrophages are a diverse set of cells that constantly shift their functional state to new metastable states in response to changes in tissue physiology or environmental challenges.
10.3 Macrophages in Wound Healing
An emerging dogma in tissue repair is that M1 macrophages are the predominant population present during the first few days after injury, corresponding to the inflammatory and early proliferative phases, whereas M2 macrophages are the primary effectors of later stages of repair or the later proliferative and remodeling phases [11, 30; Fig. 10.1]. The pattern of pro-inflammatory cytokine production by tissue-repair macrophages is perhaps the strongest evidence for the early-M1/late-M2 paradigm or at least for switching from a pro- to an anti-inflammatory phenotype.
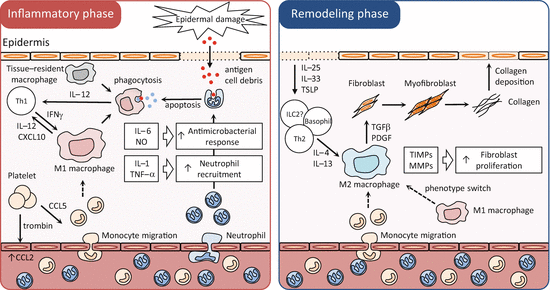

Fig.10.1
Macrophages in wound healing, different phenotypes in different phases. Schema of molecular and cellular processes with a focus on macrophages in wound healing. Tissue-resident cells initiate the immune response against exogenous antigens and recruit neutrophils from the circulation to the dermis. These recruited neutrophils trigger macrophage differentiation to M1 macrophages via antigen processing in the acute phase (inflammatory phase). If the tissue-damaging irritant persists, activated M1 macrophages can further exacerbate the inflammatory response by recruiting neutrophils. The damaged epithelial cells release alarmins, including IL-25, IL-33, and TSLP, which induce IL-4 and IL-13 production by various innate and adaptive immune cells. When the antigens are eliminated, M1 macrophage activation diminishes, and Th2-type cytokines, IL-4 and IL13, convert the inflammatory phase to the wound healing phase (remodeling phase). Th2-type cytokines differentiate M1 macrophages and recruited monocytes to M2 macrophages. M2 macrophages promote wound healing and fibrosis through the production of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and various growth factors. M2 macrophages also promote the resolution of wound healing by antagonizing the inflammatory M1 response. CCL CC-chemokine ligand, CXCL CXC-chemokine ligand, ILC innate lymphoid cell, PDGF platelet-derived growth factor
The M1 macrophage activator IFN-γ is upregulated rapidly after injury of the skin and is required for proper healing of this tissue [40, 46]. The M1 macrophage-associated cytokines IL-1β, TNF-α, IL-6, and IL-12 are expressed by macrophages during the first few hours to days after acute injury of the skin [10, 43]. Within a few days of wound debridement by phagocytic cells, the injured dermis starts to gain volume by the formation of granulation tissue. As recently reported, the dominating macrophage population in the wound at day 5 is the M2 macrophage [26] and M1 macrophages can convert into anti-inflammatory macrophages with an M2 wound-healing phenotype [5]. In contrast to pro-inflammatory and antimicrobial M1 macrophage responses, M2 macrophages exhibit potent anti-inflammatory activity and have important roles in wound healing [51].
M2 macrophages produce growth factors that stimulate epithelial cells and fibroblasts , such as TGFβ1 and PDGF [3]. TGFβ1 contributes to tissue regeneration and wound repair by promoting fibroblast differentiation into myofibroblasts , by enhancing expression of tissue inhibitors of metalloproteinases (TIMPs) that block the degradation of ECM, and by directly stimulating the synthesis of interstitial fibrillar collagens in myofibroblasts [37]. PDGF also stimulates the proliferation of activated ECM-producing myofibroblasts [41]. M2 macrophages can also regulate wound healing independently of their interactions with myofibroblasts. For example, M2 macrophages produce matrix metalloproteinases (MMPs) that control ECM turnover [49]. M2 macrophages not only recruit Th2 cells and regulatory T cells by secreting CCL17 and CCL22 [9, 19] but also serve as antigen-presenting cells that propagate antigen-specific Th2 and regulatory T-cell responses, which promote wound healing while limiting the development of fibrosis [39]. In addition, they express immunoregulatory proteins, such as IL-10, RELMα, and chitinase-like proteins, which have been shown to decrease the magnitude and duration of inflammatory responses and promote wound healing [33, 36].
Rodent M2 macrophages also express Arg1, which has been suggested to be important for tissue repair [29]. Arginine metabolism through the Arg1 pathway produces polyamines, which are important for cell proliferation, and proline, which is a major component of collagen. However, proline produced via the Arg1 pathway may not be a limiting factor for collagen synthesis [6], and the role of Arg1 in collagen production and fibrosis in vivo is complex and likely context-dependent [50]. Although Arg1 is expressed in macrophages during injury and tissue repair, Arg1 can be produced by cells other than macrophages [21]. In addition, human macrophages do not produce Arg1 [34] and therefore Arg1 appears to be derived entirely from nonmacrophage cell types in humans [6].
10.4 Macrophages in Atopic Dermatitis
Atopic dermatitis (AD) is one of the most frequent chronic inflammatory skin disorders with an increasing prevalence affecting 15–30 % of children and 2–10 % of adults in industrial countries [2]. The hallmarks of AD are a chronic relapsing form of skin inflammation accompanied by a Th2 cytokine-predominant milieu, a disturbance of epidermal-barrier function and hyperproduction of IgE to environmental allergens [4, 22].
Although emphasis has been placed on the regulatory role of T cells in AD research, recent studies indicate that macrophages are alternatively activated in the lesional skin, which may be associated with the pathogenesis of AD. In acutely and chronically inflamed AD skin, CD36 expression by macrophages is elevated [24]. In addition, macrophages expressing mannose receptors [24] and CD163 [45] are significantly increased in lesional skin of AD, compared to normal skin. Collectively, these findings indicate that alternatively activated (M2) macrophages are increased in AD skin lesions. However, it remains unclear whether alternative activation of macrophages actively contributes to the development and maintenance of chronic skin inflammation in AD.
More than 90 % of patients with AD are colonized with Staphylococcus aureus in the lesional skin [7], whereas most healthy individuals do not harbor the pathogen [38]. In addition, staphylococcal colonization density in AD patients positively correlates with the disease activity of AD skin lesion [25]. Recent study has shown that Staphylococcus δ-toxin induces skin inflammation by activating mast cells [31], indicating that increased susceptibility to skin colonization with S. aureus is a risk factor for AD. Increased susceptibility to skin colonization with S. aureus can be caused by several factors including not only skin barrier dysfunction and reduced skin lipid content, but also dysregulated innate and adaptive immune responses [22]. The TLR2 expression and TLR2-mediated production of pro-inflammatory cytokines by macrophages, which play a major role in combating S. aureus [Takeuchi et al. 1998, 46], are impaired in patients with AD [32]. In addition, the single nucleotide polymorphism R753Q in the TLR2 gene has been shown to be associated with the severity of AD [1]. Taken together, dysfunction of macrophages may exacerbate skin inflammation in AD via promoting skin colonization with S. aureus.
Although a functional disturbance of macrophages is observed in AD, no conclusive data are available on the pathogenic role of these cells in AD. Therefore, further studies are needed to clarify the precise role for macrophages in the development and persistence of AD skin lesions.
10.5 Perspectives
Macrophages are critical orchestrators of repair and regeneration in numerous tissues and may also contribute to chronic skin disorders including fibrosis and AD. Variable stimuli can induce a broad spectrum of functional macrophage phenotypes. Because this plasticity likely contributes to almost all skin diseases, especially chronic inflammatory skin diseases, to manipulate macrophage functions represents an attractive therapeutic approach. For manipulating macrophage functions properly, however, it is necessary to further understand the functional and phenotypic diversity of macrophages, by determining their proteomes and transcriptomes, as was recently performed for resident macrophages [13]. The advances in proteome and transcriptome analyses at the single-cell level may provide better understanding of a wide spectrum of macrophage activation states.
Functional regulation of macrophages by a variety of cytokine milieux complicates the precise identification of macrophage phenotypes in vivo, compared to macrophages activated in stable and polarized cytokine conditions in vitro. For example, in vivo differentiated tissue-repair macrophages do not necessarily conform to in vitro defined phenotypic categories. Although macrophages exhibit a pro-inflammatory profile in the early stage of tissue repair compared to the late stage, they may lack iNOS expression or express CD206, Ym1, and IL-10 in addition to pro-inflammatory cytokines, indicating that those macrophages do not exhibit an entire M1 phenotype. Similarly, macrophages in the later stage show an increased expression of anti-inflammatory cytokines and some M2-associated markers such as CD206, but other M2 markers, such as Ym1, may be downregulated, indicating that macrophages during the later stages of tissue repair are not entirely the M2 phenotype. Thus, tissue-repair macrophages exhibit complex and heterogeneous phenotypes that change throughout the repair process and do not correspond to in vitro defined M1/M2 categories.
Significant research effort has been concentrated on the identification of genetic factors and dysregulated immunologic pathways that could lead to the manifestation of AD. Although the pathogenic roles of various immune cells such as T cells, eosinophils, and mast cells in AD have been intensely investigated, much less is known about how macrophages contribute to the chronic skin inflammation in AD. Because of their possible versatile roles in the pathophysiology of AD, their multifaceted character, and their capacities to both promote and prevent the manifestation of allergic skin inflammation, macrophages represent promising therapeutic targets for AD in the future as well as for cancer, fibrosis, and multiple sclerosis [17].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








