Fig. 20.1
Solitary nodular chest wall recurrence 3 years after a mastectomy
In the presence of postirradiation fibrosis, early diagnosis of local recurrence may be challenging. Recurrences appear as macular erythema, pruritic or no pruritic, or small papules, or hyperpigmented superficial plaques with surrounding areas of erythema (Fig. 20.2). If the skin lesions had appeared 1 month earlier and had not improved with over-the-counter topical preparations applied by the patient, biopsy is mandatory.
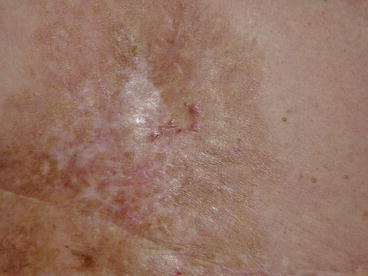

Fig. 20.2
Hyperpigmented superficial plaques with surrounding erythema, a kind of biopsy-proven recurrence appeared about 1 year after a mastectomy followed by radiation therapy
Loco-regional failures may include lump or swelling of lymph nodes in the axilla or in the supraclavicular fossa. The presence of a palpable mass in the axilla or in the supraclavicular fossa should be considered a tumour recurrence until otherwise proven. In some cases, patients may present with a suddenly occurred lymphoedema, pain in the arm and shoulder or increasing sensory or motor loss in the arm or hand. These latter symptoms are often the result of brachial plexus involvement.
Internal mammary recurrences are rare and often present with a deep, fixed mass in an intercostal space next to the ipsilateral sternal border. These recurrences often first present with pain and tenderness, without noticeable mass, and it may be difficult to differentiate them from sternal metastases.
Differential diagnosis. The clinical significance of distinction between an IBTR as a recurrence of the initial tumour and a new primary tumour arising in the breast remains uncertain and in many cases misleading. The differential diagnosis is generally made on clinical grounds, such as the distance of new tumour from the site of the initial tumour, and on disease-free interval. Several series have found that survival decreased with local failures in the same location as the initial tumour (compared to elsewhere in the breast), but others have not. However, this may be related to the longer interval to failure for the recurrences elsewhere in the breast rather than the location itself.
Clinical differential diagnosis should be made with postradiation or postoperative change, foreign body cyst around suture material, cellulitis, severe inflammation of dermal and subcutaneous layers of the skin, fat necrosis from trauma or lipofilling, bony nodule on a rib or costal cartilage from surgical trauma. In patients who had postoperative radiation therapy, a radiation-induced angiosarcoma could be rarely observed, typically late with a median interval of 10 years posttreatment.
20.1.3 Role of Imaging
Mammography. The role of mammography after BCT is close surveillance of the treated breast for clinically occult recurrent BC as well as screening of the contralateral breast. Patients typically undergo their first posttreatment mammogram 6–12 months after completion of radiation treatment. The degree of oedema and distortion that is seen on the mammogram can vary significantly from one patient to another. At the time of the baseline mammogram after completion of treatment, some patients will have extensive oedema, trabecular thickening and architectural distortion, while other patients will have minimal mammographic changes. These posttreatment changes tend to be maximal at about 6 months after treatment and may pose a challenge to the breast imager in detecting recurrent disease. On subsequent mammograms, the posttreatment changes may remain stable but usually decrease in prominence over time. Therefore, any new or worsening finding on subsequent screening mammography, such as new calcifications, new mass or increasing architectural distortion, needs to be viewed cautiously and evaluated carefully for recurrent disease [10].
Ultrasound. For the evaluation of new or suspicious mammographic masses as well as clinically palpable findings, breast ultrasound is an essential tool that plays a complementary role to mammography and physical examination. In the evaluation of palpable breast masses, sonography is particularly helpful, since the mammographic evaluation can be limited due to the posttreatment changes. In patients presenting with palpable breast masses, prospective and retrospective studies have reported the negative predictive value of combined mammography and sonography to be very high, approaching 100 %. In addition, if a lesion is visible on ultrasound, a minimally invasive core-needle biopsy or fine-needle aspiration can be performed under sonographic guidance [11].
Breast MRI is well known to detect mammographically occult BC. Unlike mammography and sonography, MRI dynamically evaluates the breast tissue. In addition to assessing the morphology of the breast lesions, the vascularity of any lesions may be evaluated after intravenous contrast enhancement (see Sect. 5.3). Lesion enhancement is based on the number and the permeability of the blood vessels.
There is, however, some overlap in enhancement characteristics seen in benign and malignant lesions. In women with equivocal mammographic findings after breast-conservation treatment, the enhancement pattern on contrast-enhanced MRI can be helpful in differentiating posttreatment scar from recurrent cancer. Breast MRI can also detect mammographically occult local recurrence in the breast BCT [12, 13].
20.1.4 Workup
Since local or regional recurrence is a marker of increased aggressiveness, all patients experiencing a relapse, both local and regional, have to undergo proper systemic workup to rule out the presence of synchronous distant metastases. In addition to a complete history and physical exam, patients should have a CT of the chest/abdomen/pelvis and a bone scan. On the other hand, CT should be considered in any patient with new onset pain, paralysis, paraesthesia or lymphoedema, even in the absence of palpable disease. PET scans are used in uncertain situations or when the results of the CT or bone scan are equivocal.
For a woman, learning that she has a recurrent BC may be harder than dealing with the initial diagnosis. An appropriate intervention, including psycho-education, is needed for patients diagnosed with first recurrence in order to detect and manage psychological distress.
20.2 Treatment of Isolated Local or Regional Recurrence
Clinical Practice Points
In a patient previously treated for an invasive BC, local recurrence presents a unique challenge to the oncologist because there is a paucity of randomised studies to guide in choosing the optimal combination and sequence of therapies.
The management of each patient requires a multidisciplinary approach that depends not only on factors specific to the recurrence itself but also on factors related to the original treatment.
Durable local salvage is important in preventing the consequences of uncontrolled loco-regional disease.
Wide local excision of all gross disease is recommended with the purpose of both maximising subsequent local control and moderating the required dose of chest wall irradiation.
Chemotherapy significantly increases overall and disease-free survival, particularly in receptor-negative patients.
20.2.1 Local Treatment
Local recurrence after mastectomy. Patients with isolated chest wall recurrence after mastectomy should be treated with wide local excision of the relapse after complete local and systemic workup. This is also recommended for multiple nodules amenable to resection with negative margins.
Postoperative loco-regional radiotherapy is added for those patients who did not receive it after primary treatment. For isolated chest wall recurrences, the supraclavicular nodes should be electively treated concurrently with the entire chest wall because of the increased risk of subsequent relapse without radiation. Elective irradiation of a clinically uninvolved axillary or internal mammary node region is not necessary [14].
Ipsilateral breast tumour recurrence (IBTR). Up to now, IBTR after BCS has been almost exclusively treated by mastectomy even in patients with small and late recurrences [15, 16]. The number of published studies reporting the outcome of patients treated with conservative surgery alone is limited. There are only retrospective small series, which compared repeat lumpectomy and mastectomy.
Kurtz et al. [17] reported a series of 50 patients with stage I or II BC treated with breast-conserving surgery and radiation who subsequently underwent wide local excision for a clinically isolated IBTR, with or without axillary recurrence. Of the recurrences, 80 % were less than 2 cm in size, 62 % were in the vicinity of the original tumour, and all were without skin involvement. The second local failure rate in the salvaged breast was 38 % at 5 years, with a 5- and 10-year survival of 67 and 42 %, respectively. The only significant factors for local control on multivariate analysis were a disease-free interval greater than 5 years (92 % vs 49 %) and negative resection margins (73 % vs 36 %).
Salvadori et al. [18] reported that the risk of further local recurrence after BCS was higher compared with mastectomy (19 % vs 4 %). While the most published studies recommend a second conservative approach only in selected groups with small and late recurrences, a large observational analysis published by Chen and Martinez [19] discouraged the use of lumpectomy for all patients with ipsilateral breast recurrence. In fact the author found that the group of patients undergoing BCS had significantly worse overall survival compared to the mastectomy group (67 % vs 78 %).
Despite this data subsequent and current studies have shown encouraging results with the use of lumpectomy alone. The largest and most recent series has been published by Gentilini et al. [20]. This retrospective study evaluated 161 patients who underwent second BCS in an attempt to identify the best candidates for BCS. The 5-year OS was 84 % and 5-year cumulative incidence for a further local recurrence was 29 %. Tumour size and time to breast recurrence <48 months increased significantly the risk of second local relapse.
A repeat BCT demands tumour-free margins and an interstitial brachytherapy. Despite that, the indication for second lumpectomy is restricted for suited patients (small size, low risk). As data from prospective randomised clinical trials are missing, an impaired regional tumour control (without disadvantages for overall survival) cannot be ruled out completely. Systemic therapy after resected local recurrence (re-adjuvant) is associated with improved disease-free and overall survival.
Endocrine treatment in hormone-sensitive tumours improves disease-free survival. The impact on overall survival is still debatable. Repeat irradiation breast for recurrent BC is feasible. If no prior radiotherapy has performed after BCS, whole-breast radiation should be performed.
There has been limited experience with repeating radiation therapy for an IBTR following breast-conserving surgery and whole-breast radiotherapy. Deutsch [21] reported on 39 patients who received initial radiation doses of 45–50 Gy to the breast (with or without a boost) and who were treated for local recurrence with repeat lumpectomy and re-irradiation. The prescribed re-irradiation dose was 50 Gy in 25 fractions using electrons. The rate of second local recurrence was 29 % (8/39). Overall cosmetic outcome was excellent or good for 75 % (27/36) of the evaluable patients. Trombetta et al. [22




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








