Fig. 23.1
Schematic representation of a liposome
Particularly, liposomes have been shown to be a promising skin drug delivery system: their use may produce severalfold higher drug concentrations in the epidermis and dermis and lower systemic concentrations when compared to conventional dosage forms (El Maghraby 2008). Clearly, their topical use depends on their characteristics as size, surface, charge and chemical composition. Mezei and Gulasekharam in 1980 were the first to employ liposomes as skin drug delivery systems: they demonstrated that vesicles of dipalmitoylphosphatidylcholine (DPPC) and cholesterol (CH) (1.1:0.5, molar ratio) increased the concentration of triamcinolone acetonide in the epidermis and dermis by four- to fivefold and reduced percutaneous absorption compared with a standard ointment (Mezei and Gulasekharam 1980). Several mechanisms have been suggested for liposomes acting as skin drug delivery systems. According to the free drug mechanism, the drug may permeate the skin independently after exiting from the vesicles, since the liposomes themselves enhance the transdermal drug delivery by lowering the permeability barrier of the skin, changing the ultrastructures of the intercellular lipids and the enthalpy of the lipid-related transitions of the stratum corneum (Bernadete et al. 2011). In some cases, the vesicles may adsorb to the stratum corneum surface with subsequent transfer of drug directly from vesicles to the skin (El Maghraby et al. 2008). Moreover, vesicles have been reported to fuse and mix with the stratum corneum lipid matrix, increasing drug partitioning into the skin (El Maghraby et al. 2008). The possibility that intact vesicles penetrate human skin acting as carriers and go deep enough to be absorbed by the systemic circulation has been also suggested (El Maghraby et al. 2008).
Different novel vesicular systems derived from liposomes have been proposed as a valid alternative and among these, niosomes, nonionic surfactant-based vesicles, represent one of the most suitable options: niosomes appear to be similar in terms of their physical properties to liposomes, but they are preferred in topical delivery because of chemical stability and low cost of production (Schreier and Bouwstra 1994).
23.1.2 Gel Systems
The term gel was introduced in the late 1800s to name semisolid systems in which a liquid phase is constrained within a three-dimensional polymeric matrix of natural or synthetic gums with a high degree of physical or chemical cross links, as shown in Fig. 23.2 (Narin 1997). Gel forming polymers include natural polymer (proteins and polysaccharides), semisynthetic polymers (cellulose derivatives), synthetic polymers (carbomers and poloxamers) and surfactants (cetostearyl alcohol and polyoxyethylene glycol alkyl ethers (Brij™, Croda International PLC)). Gel also possess a degree of flexibility very similar to natural tissue, and due to their high water content, they resemble natural living tissue more than any other type of synthetic biomaterial. Moreover, they have significant roles in pharmaceutical and cosmetical fields because of their biocompatibility, non-toxicity and good skin adhesion (Peppas 1986). Additionally, their insoluble cross-linked structure allows medium for dissolution of hydrophilic drugs, and since only the dissolved drug presented to the skin is able to enter the stratum corneum, gels could represent a strategy to enhance the percutaneous drug absorption and release across the skin in well-defined specific manner (Wichterle and Lim 1960).
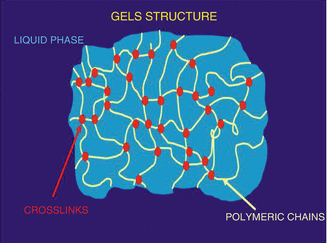

Fig. 23.2
Schematic representation of a gel system
Clearly, the type of vehicle used to formulate a topical dermatological product greatly influences its effectiveness. Gels prepared with organic polymers, such as carbomers, impart an aesthetically pleasing, clear, sparkling appearance to the products and are easily washed off from the skin with water; vehicles containing large amounts of oleaginous substances provide an emollient effect to dry irritated skin; bases made up of non-volatile oleaginous substances can form an occlusive barrier on the skin that prevents the escape of moisture from the site of application, causing hydration of the stratum corneum and increase of opening up of intra- and intercellular channels for easier passage of drug molecules (Hoare and Kohane 2008).
23.2 Liposomal Gel Systems
23.2.1 Introduction
Taking into account all the previously mentioned reasons, the incorporation of vesicular systems into a gel dosage form has been designed as a new strategy to improve drug percutaneous permeation: the resulting multicomponent systems, named liposomal gel (Fig. 23.3), may possess the advantages of the individual formulations (vesicular suspensions and gel systems) and some other important benefits (Foldvari 1996). Since topically applied liposomal suspensions may leak from the application site, they could be mixed with gels in order to obtain semisolid formulations. In addition, liposomal gels were found to enhance the skin retention of drugs and to provide higher and sustained skin concentrations of therapeutics compared to conventional gels and creams, without enhancing their systemic absorption (Pavelic et al. 2005). In addition, the stability of the liposomes (membrane integrity and mechanical stability) has been reported to increase when incorporated into a gel matrix (Mourtas et al. 2007). Additionally, liposomal gels have been demonstrated to have better rheological characteristics with respect to the liposomal dispersion (easiness of application and removal from the skin), to ensure an appropriate release of the active principle (compatibility with most active substances) and increased skin tolerance and compliance for patients. Clearly the type and concentration of the polymer forming the gel matrix has been reported to influence the stability and release rate of the active substance, whereby an assessment of the physico-chemical properties of the drugs, liposomes and polymeric gel must be made to avoid adverse effect and chemical, physical and biological incompatibility (Mourtas et al. 2008).
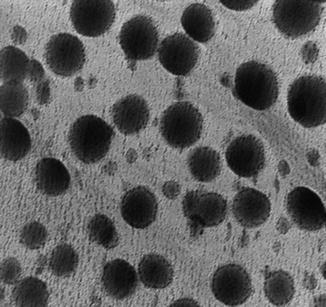

Fig. 23.3
Photomicrographs of a vesicles-gel system as seen by transmission electron microscopy (TEM) (Adapted from reference Antunes et al. 2011)
Moreover, liposomes in a gel can be more stable to environmental stimuli compared with bare liposomes in a dispersion, and when a drug is placed inside the liposomal core and the liposomes are included in a gel network, the drug will experience a combination of transport resistances due to the liposomal bilayer and the network itself: this results in a release of the drug over a longer period of time. Additionally, this can also avoid the problem of “burst release” seen with some polymer gels where a large bolus of drug is released initially from the gel, which can cause toxicity (Lee et al. 2012).
The first study on the incorporation of liposomes in a gel dosage form was reported by Mezei and Gulasekharam in 1982 (Mezei and Gulasekharam 1982). They compared the permeation of triamcinolone from plain and liposomal gel and they found that the application of the liposomal gel resulted in a concentration of triamcinolone acetonide approximately five times higher in the epidermis and three times higher in the dermis, than application of the conventional drug gel. The results of this study and those reported earlier by the same researchers (Mezei and Gulasekharam 1980) suggested the inherent potential of liposomes (when applied in a gel) as a drug delivery system for cutaneous application and the role of liposomes in the formation of a large drug reservoir in the skin, which is useful in local treatments. Since then, a lot of researchers explored the potential of liposomal gel systems in transdermal drug delivery (Gabrijelcic and Sentjurc 1995).
The most used polymers to obtain liposomal gels are carbomers, cellulose derivatives and poloxamers. Carbomers are polymers of acrylic acid cross-linked with polyalkenyl ethers or divinyl glycol. They swell in water up to 1,000 times their original volume to form a gel when exposed to a pH environment above 4.0–6.0. Because the pKa of these polymers is 6.0–0.5, the carboxylate groups on the polymer backbone ionize, resulting in repulsion between the negative charges, which adds to the swelling of the polymer (Florence and Pu 1994). Carbomer polymers are very well suited for aqueous formulations of the topical dosage forms: many commercial products available today have been formulated with these polymers, as they provide numerous benefits to topical formulations. Carbomer polymer possesses low toxicity and low irritancy potential and they are non-sensitizing even upon repeated usage. In addition, due to their extremely high molecular weight, they cannot penetrate the skin or affect the activity of the drug. Because of their excellent thickening, suspending, emulsification and suitable rheological properties, good tissue compatibility and convenience in handing and ease of application, carbomer gels represent a good alternative to oil-based formulations (Jian Hwa 2003).
Cellulose polymers (hydroxyethyl cellulose, hydroxypropyl cellulose, sodium carboxymethyl cellulose) are examples of polymers that have been reported to possess adhesive properties (Jones et al. 1997). Chemically, these linear polymers are cellulose derivatives possessing various degrees of substitution and may be ionic or nonionic; following addition to an aqueous phase, these cellulose derivatives undergo swelling prior to dissolution. Pharmaceutically, water-soluble cellulose polymers have found widespread applications, e.g. in the formulation of solid dosage forms, aqueous disperse systems as viscosity enhancing agents and in products for topical application (Peppas et al. 2000).
Poloxamers are polymers consisting of a relatively long hydrophobic poly(propylene oxide) (PPO) middle block and two hydrophilic poly(ethylene oxide) (PEO) end blocks and are commercially available as Pluronics® (BASF, Hanover, Germany). In the presence of a either solvent selective for the hydrophilic PEO blocks, such as water, PEO–PPO–PEO block copolymers self-organize into a variety mesophases with lamellar, hexagonal or cubic structure (Batrakova and Kabanov 2008). Pluronics® have attracted particular interest in the design of dermal and transdermal delivery systems, with a view to promoting, improving or retarding drug permeation through the skin. Moreover, they possess specific pharmacological actions, in particular the dynamic PEO chains prevent particle opsonization and render them ‘unrecognizable’ to reticuloendothelial system (RES) and macrophages (Tavano et al. 2010). Several studies reported the advantageous interactions between Pluronics® and liposomes: in particular, these polymers have been used to sterically stabilize the vesicles and, hence, to prolong their half-life after parenteral administration (Kostarelos et al. 1999).
23.2.2 Overview on Liposomal Gel Systems Used for Dermal Drug Delivery
A summary of recent works in the area of novel liposomal gel formulations for dermal drug delivery is provided below.
Pavelic et al. in 2001 developed a liposomal gel system able to provide sustained and controlled release of calcein, as model drug, for local vaginal therapy (Pavelic et al. 2001). Traditional liposomes were prepared from egg phosphatidylcholine (EPC) and egg phosphatidylglycerol sodium (EPG-Na); they were incorporated in gels of polyacrylate, i.e. carbomer gels (Carbopol 974P NF or Carbopol 980 NF, BF Goodrich, Belgium). In vitro release of encapsulated calcein from both liposomal gels was tested and compared with that of liposomes dispersed in buffer (control). A slower release of calcein from liposomal gel was achieved: in fact, after 24 h, more than 80 % of the originally encapsulated calcein was retained in liposomes embedded in gel with respect to the control (60 %, respectively). The authors ascribed these results to the increased viscosity of the gel system which reduced migration of drug molecules, acting as drug reservoir system, while preserving the structure and integrity of liposomes.
Similar results were obtained by Glavas-Dodov and collaborators in 2002 (Glavas-Dodov et al. 2002). The authors compared the in vitro drug release properties of free and liposomally entrapped lidocaine hydrochloride hydrogels. As expected, hydrogel formulations showed higher release rate of lidocaine hydrochloride compared to liposomal gel. Moreover, the release kinetic in the case of liposomal gels can be described as diffusion controlled, while a steady-state release, achieved after the third hour, suggested that liposomes act as a reservoir system for continuous delivery of the drug and for these reasons they could have a potential as dermal delivery systems with prolonged and sustained drug release.
Another delivery system based on liposomal gels containing vitamin E acetate was designed in 2006 by Padamwar and collaborators to improve topical drug delivery (Padamwar and Pokharkar 2006). The prepared liposomal dispersion showed sevenfold increase in drug deposition in rat skin compared to the control (plain drug dispersion), and the liposomal gel formulation demonstrated sixfold and fourfold increase in drug deposition in rat skin compared to the control gel and marketed cream, respectively. Moreover, the liposomal gel formulation was found to be more stable than the corresponding liposomal in terms of drug entrapment efficiency and uniformity up to 3 months.
Mura et al. in 2007 designed and evaluated the potential of a liposomal gel formulation for the topical delivery of benzocaine as a model drug (Mura et al. 2007). Drug permeation from liposomal dispersions (based on mixtures of phosphatidylcholine, cholesterol, ethanol and water) as such or formulated in a carbomer gel was evaluated both through artificial lipophilic membranes and excised abdominal rat skin, whereas in vivo anaesthetic effect was tested in rabbits. Liposomes were prepared with drug encapsulated in the hydrophilic core or incorporated in the hydrophobic bilayer. The results of the benzocaine release study across artificial membranes showed that the presence of the polymeric network in the case of liposomal gels gave rise to a general reduction of the drug permeation rate and allowed obtainment of a more regular release profile as a function of time, with respect to simple liposomal dispersions. Interestingly, drug-loaded gels showed a faster drug release in respect to the gel-containing liposomes with the drug in the lipophilic phase, but slower than that obtained from liposomal gels with the drug encapsulated in the aqueous phase. In permeation studies using rat skin, a higher reduction of drug permeation rate was noted, due to the more complex permeation process through rat skin than across artificial membranes. In percutaneous permeation studies across rat skin, the difference between the use of the liposomal dispersion as such or formulated in the carbomer gel was less evident, probably due to the major controlling effect exerted by the skin on the drug permeation rate. Moreover, an initial lag phase was present in respect to the permeation studies thought artificial membranes, which was attributed to the longer time necessary to saturate the skin membrane and to reach a pseudo steady-state flux condition between donor and receiver compartments. Finally, the lowest drug permeation was observed from the conventional gel in comparison to all liposomal gel formulations (containing drug concentrations ranged from 0.05 to 0.5 % w/w), confirming the hypothesized permeation enhancing effect of liposomal vesicles on drug delivery, which cannot be efficiently estimated by using the artificial membrane.
Mourtas et al. in 2007 evaluated the effect of liposomes, drugs and gel properties on drug release kinetics (Mourtas et al. 2007). They studied the release of two model compounds, one hydrophilic (calcein) and one lipophilic (griseofulvin, GRF), when dissolved directly in hydrogels (control gels) or dispersed in hydrogels in the form of liposomes (liposomal gels). Drug-loaded liposomes based on PC or DSPC/Chol were dispersed in carbomer, i.e. Carbopol® 974 (Chemix S.A, Athens, Greece), HEC (Natrosol 250 HX, Hercules Inc, Athens, Greece) or a mixture of these two hydrogels. Results demonstrated that depending on the intended use of a liposomal gel formulation, the first parameter that should be considered is whether the drug is hydrophilic or lipophilic. In fact, the release of calcein from liposomal gels was slower compared to that obtained from conventional gels and strongly dependent on the liposome-membrane rigidity. If the drug was encapsulated in DSPC/Chol liposomes and dispersed in gels, it was released significantly slower compared to the corresponding formulation based on PC liposomes. In the case of GRF, the release from liposomal gels was determined by drug loading. At high drug loading levels, GRF is released steadily from liposomal gels irrespective of liposome type (PC or DSPC/Chol). Moreover, in the case of the lipophilic drug, liposomes provided means for substantially increased drug loading in gels acting as reservoirs which released the drug in a sustained manner. Finally, the authors demonstrated that in the case of liposomal carbomer (Carbopol®) gels that behave predominantly as elastic solids and have substantially different flow properties (compared with HEC-based gels), increased release rates for free calcein and GRF, indicating easier diffusion of the compounds through this system, were obtained.
In the following year, Mourtas et al. investigated the effect of added liposomes on the rheological properties of a hydrogel containing 0.40 % w/v of carbomer, i.e. Carbopol® 974 NF (Chemix S.A., Athens, Greece) and 1.5 % w/v of HEC (Mourtas et al. 2008). PC or HPC liposomes, plain or mixed with Chol, were used to prepare liposomes. As reported by other authors, the lipid composition of liposomal bilayers was found to strongly influence the rheological properties of liposomal gels. Zero-rate shear viscosity and power law index values revealed that addition of PC liposomes to the hydrogel had the smallest effect on its rheological properties, even when the highest lipid concentration was used (20 mg/ml). Oppositely, incorporation of HPC (or HPC/chol) liposomes into gels resulted in a significant increase of the elastic character of the gel, which increased with increasing lipid concentration. Since drug rate permeation was found to be dependent on gel viscosity, authors demonstrated that liposomal gel based on HPC could be used to act as drug reservoir and to ensure a slow drug release over a prolonged time period, while liposomal gel based on PC could be used to obtain faster drug permeation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree