Fig. 7.1
Annular plaque with vesicles at perimeter of lesion
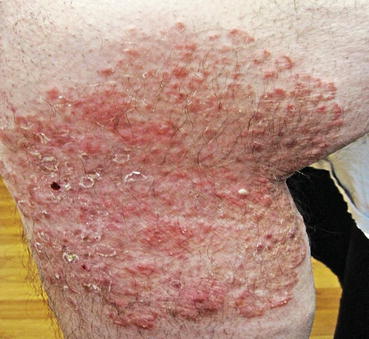
Fig. 7.2
Papulovesicular variant
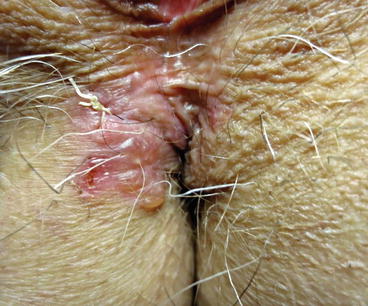
Fig. 7.3
Involvement of the perineal area
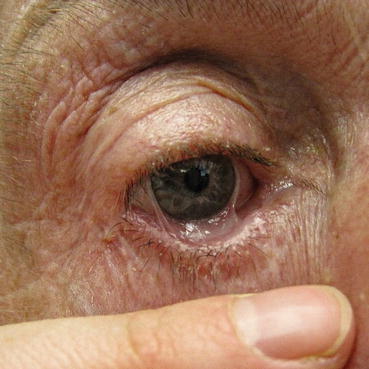
Fig. 7.4
Ocular involvement showing symblepharon and foreshortening of the inferior fornix
Diagnosis
The gold standard test for diagnosis is a biopsy of perilesional, clinically normal-appearing skin or mucous membrane immediately adjacent to a lesion that reveals linear IgA deposits along the basement membrane zone on direct immunofluorescence (DIF) testing [3]. The additional presence of linear IgG deposition along the basement membrane has provoked some controversy. Some clinicians have said that IgA alone should be present at the BMZ whereas others have said that IgA and IgG may be present, but IgA must be predominant [4]. A Japanese review of 213 patients with LABD found both antibodies in approximately 20 % of the cases [5]. We prefer the designation of linear IgA/IgG bullous dermatosis (LAGBD) when both are present [6].
The histopathologic findings with hematoxylin and eosin staining of involved skin are identical to those of dermatitis herpetiformis, showing lymphocytes, occasional eosinophils, and neutrophilic dermal papillary microabscesses [3, 7]. Additionally, another characteristic finding is a subepidermal blister with a diffuse underlying neutrophilic infiltrate in the dermis (Fig. 7.5).
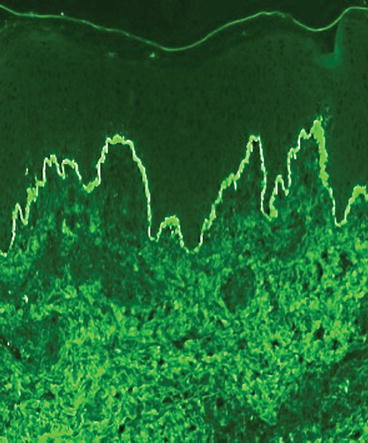

Fig. 7.5
Direct immunofluorescence of IgA in the basement membrane zone
LABD is divided into subtypes based on ultrastructural location of IgA – a sublamina densa type and a lamina lucida type. These types can be identified using BMZ salt–split human skin and indirect immunofluorescence. Separation occurs in the lower lamina lucida. Lamina lucida antigens will adhere to the epidermal side of the basement membrane separation, whereas lamina densa and sub-lamina densa antigens will bind to the dermal side. If the patient has positive IgA BMZ antibodies on indirect immunofluorescence with BMZ salt–split skin, the sub-type can be identified (Fig. 7.6). In cases without circulating IgA basement membrane antibodies, immunoelectron microscopy or basement membrane separation of the DIF positive biopsy can identify the type. In most cases this is unnecessary, since treatment for both variants of LABD is the same; however, it is the authors’ experience that patients with sub-lamina densa antigens are more resistant to treatment. Furthermore, in both situations antibody titers by indirect immunofluorescence correlate well with response to therapy.
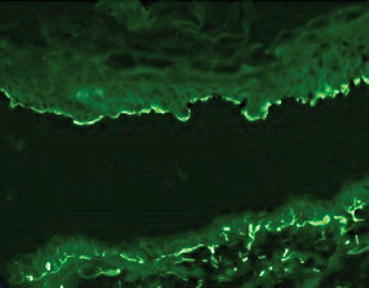

Fig. 7.6
Indirect immunofluorescence of IgA binding to epidermal side in salt-split skin
The lamina lucida type predominantly targets a 97-kDa antigen and a 120-kDa antigen that are the proteolytic fragments of the extracellular portion of the bullous pemphigoid antigen 2 (BP180), a key epidermal-dermal adhesion transmembrane protein [8, 9]. Less frequently, LABD is associated with the NC16A epitope of BP180 [10]. The sub-lamina densa type of LABD has been reported to be predominantly Type VII collagen, although a number of other antigens have been proposed [11].
Epidemiology
Reported incidence rates range from less than 0.5–2.3 cases per million individuals yearly. No predilection based on ethnicity or gender for LABD has been established [7]. LABD rarely occurs in neonates [12]. It can develop in children between the ages of 6 months and 10 years. The average age of onset in 25 affected children was 4.5 years [13]. Childhood cases frequently remit spontaneously in 12–36 months. Adults generally present with LABD later in life, with many cases occurring after age 60. Lastly, drug-induced cases may occur at any age.
The etiology of LABD may be drug-induced or idiopathic. Vancomycin is the most common offending medication; however, over 20 other medications have been incriminated. Beta-lactams and cephalosporins are other inciting antibiotic classes. Non-steroidal anti-inflammatory medications and angiotensin-converting enzyme inhibitors, such as captopril, have been cited in case reports. Some clinicians have said that the idiopathic form of LABD does not differ significantly from the drug-induced form [7]. Both forms of LABD can present exclusively with localized involvement rather than with widespread distribution. They may or may not involve the mucosae. Other clinicians have indicated that the drug-induced form is usually more severe and can even resemble erythema multiforme or toxic epidermal necrolysis [14].
Adults are more frequently afflicted with drug-induced LABD; however, it has also been reported in children [7]. The onset of lesions generally begins within the first month of drug administration and then they resolve gradually over the ensuing several weeks; however, lesions may persist beyond this timeframe in some patients. Furthermore, if the patient is re-exposed to the inciting medication, rapid reappearance of the lesions can occur.
Associations
The most common disorder associated with LABD is ulcerative colitis (UC) [5]. Paige et al. found that of 70 LABD patients, 5 patients (7 %) had UC preceding the diagnosis of LABD by an average of 6 years [15]. A review from Japan of 213 cases of LABD found 4 patients with UC [5]. It is believed that chronic inflammation in the colon exposes BMZ antigens, creates conformational neoantigens, and stimulates the mucosal immune system (IgA) to react to these autoantigens. Some patients have complete resolution of their disease after colectomy, whereas others have persistent disease or recurrences after removal of the colon [16, 17].
Malignant disorders – lymphoproliferative and solid organ type – have also been associated with LABD in multiple case reports [18–23]. Despite these reports and many others, no retrospective analyses have been performed to confirm this association.
Prognosis
Idiopathic LABD can persist from months to several years in adults, whereas in children, it typically resolves before puberty [13, 27, 28]. This disease can also prevail for a decade or even longer in some patients. It can also recur after long periods of remission [27]. This circumstance is in contrast to drug-induced LABD, which usually improves within a few days of cessation of the offending drug and resolves within several weeks [29].
The treatment duration for idiopathic LABD is variable. Therapy is generally continued for several weeks after complete resolution of lesions and then gradually tapered off. If at any time lesions recur, the treatment medication must be restarted [27]. Cutaneous lesions typically heal without scarring; however, mucosal lesions may lead to stricture formation or conjunctival and corneal scarring. These sequelae can have a significant impact on patients’ oral hygiene and nutritional support.
Treatment
Predisposing Factors
In view of the multiple contributing factors that may be operative in an individual case, several questions must be answered as they will determine the eventual therapeutic approach.
Childhood Disease
Preschool children presenting with idiopathic childhood disease will usually experience remission of symptoms in several months to years. Recommendations are to suppress symptoms, usually with dapsone, as intensive immunosuppression therapy is seldom necessary.
Drug-Induced Disease
Separation of drug-induced LABD from idiopathic disease on the basis of clinical exam alone is virtually impossible. A careful history of medication use must be taken with special attention to medications started within the last month. If a medication is implicated, termination of that medication is very likely to produce complete resolution within weeks using only suppressive therapy with dapsone. In severe or persistent cases prednisone may be used to achieve faster resolution. Therapy should be tapered early in the treatment course, within 4–6 weeks, to ascertain whether the disease is still active, warranting continuation of systemic therapy. A prolonged treatment course is rarely necessary.
Associated Disorders
Ulcerative colitis. Determining the presence of inflammatory bowel disease symptoms such as those associated with ulcerative colitis is essential. Treatment of the underlying inflammatory bowel disease, whether it be with medical or surgical treatment, is likely to improve or clear LABD.
Malignancy. Age-appropriate screening for malignancy is advised with special attention to signs and symptoms of lymphoma, including fevers, chills, night sweats, and weight loss. If identified, treatment of the underlying malignancy is likely to improve or clear the LABD.
Mucosal Disease
Ocular disease. The greatest morbidity in LABD is related to scarring mucosal disease, especially in the eye. Clinicians must examine the conjunctivae for the presence of erythema, symblepharon, and foreshortening of the inferior fornix [13]. If conjunctival disease has manifested, this warrants an ophthalmologic evaluation as well as the initiation of aggressive treatment with immunosuppressants or rituximab or both.
Oral and esophageal disease. Oral and pharyngeal involvement requires evaluation for disease by otolaryngology and gastroenterology. These circumstances may require dilatation of secondary constrictions during and after aggressive systemic treatment.
Additional IgG Antibodies
The additional presence of IgG antibodies is probably associated with resistance to dapsone, and indicates the need for additional systemic treatment with corticosteroids and immunosuppressives.
Systemic Treatment
Dapsone
Dapsone is the first-line medication therapy for LABD [28, 30]. Baseline glucose-6-phosphate dehydrogenase levels must be evaluated to ensure that the oxidative stress of dapsone will not induce a hemolytic crisis. Baseline complete blood count with auto-differential and liver function tests (LFTs) should be also evaluated. Dapsone is started at a low dose: 25–50 mg daily in adults and generally less than 0.5 mg/kg daily in children. This dose is gradually titrated upward over several weeks, depending on tolerance and treatment response. Complete blood counts (CBC) must be monitored every 2–4 weeks for the first 3 months to evaluate for leukopenia or severe hemolysis. Hemolysis to some degree occurs in all patients. Elderly patients or those with cardiac compromise need to be monitored closely as small decreases in the hematocrit or hemoglobin or minimal methemoglobinemia may produce symptoms [31]. Treatment response may occur in 24–48 hours with resolution of lesions within a few days of initiating therapy. If the disease is completely controlled with dapsone, a systematic approach at tapering is recommended. Dapsone should be tapered no more frequently than every 1 to 2 weeks by 12.5–25 mg. Development of an occasional small lesion is not an indication for increasing the dapsone dose as such lesions can be treated topically with potent corticosteroids. Tapering dapsone slowly will eventually allow discontinuation of treatment in cases that undergo spontaneous remission with time. Some patients with more extensive disease, particularly scarring mucosal disease and incomplete response to dapsone therapy may need oral corticosteroids or immediate treatment with rituximab to accelerate improvement of symptoms and effectively suppress the lesions [32, 33].
Sulfapyridine
For patients who are intolerant of dapsone, there are other sulfa-based drugs available: sulfasalazine and sulfapyridine. Although there is no published verification of therapeutic efficacy, patients who are allergic to dapsone or sulfapyridine can be given a trial of the alternate medication at a low dose; and they are generally able to tolerate it. Sulfapyridine is not commercially available in the United States, but can be obtained through compounding pharmacies. Sulfapyridine dosing should be started at 0.5 g 3 times daily and increased up to 6 g daily for control of symptoms. Sulfasalazine is metabolized to sulfapyridine in the intestine; however, the active metabolite level is more predictable when sulfapyridine, itself, is given. Sulfasalazine should be dosed between 1 and 2 g daily [34]. Both medications have the potential adverse effects of agranulocytosis and hypersensitivity reactions, but not hemolysis. Adequate fluid intake and possible alkalinization of the urine with oral bicarbonate is also recommended to reduce the risk of drug-induced nephrolithiasis. As with dapsone, lab monitoring (CBC, LFTs and urinalysis) is recommended periodically.
Oral Corticosteroids
As stated above, those patients with more extensive disease and incomplete response to dapsone may need oral corticosteroids (1 mg/kg daily) for faster resolution of symptoms and effective suppression of the lesions [32, 35, 36]. Corticosteroids may be particularly effective in patients who also have IgG BMZ antibodies on direct immunofluorescence or in the serum. Patients with lone IgA antibodies may not respond to corticosteroids at any dose.
Colchicine
Colchicine can be effective in children with LABD. Some case reports and case series have demonstrated it as a reasonable substitute therapy for dapsone [35, 37–39]. In a series of eight children with systemic glucocorticoid-refractory LABD, the addition of colchicine led to dramatic improvement in 5 patients within 4–6 weeks. Furthermore, these children were able to taper off steroid therapy. The typical dose in children is 0.6 mg twice daily. According to some case reports, adults have also responded to colchicine; however, other authors have not seen such reported results. The adult dosage is 0.6 mg taken 3 to 4 times daily. The dosage is most often limited by gastrointestinal side effects, (especially diarrhea), secondary to the higher dosages of colchicine.
Tetracycline and Niacinamide
This therapeutic combination has been effective for the treatment of bullous pemphigoid and has been applied to LABD. Three adult patients with LABD reported disease resolution within a few weeks of starting therapy [40–42]. The dosing range for tetracycline and nicotinamide are 1000–1500 mg daily and 900–2000 mg daily, respectively. Children under age 9 cannot take tetracycline due to the adverse effect on developing teeth.
Other Antibiotics
Children with LABD may respond to systemic antibiotic therapy. The mechanism for efficacy is not known; whether it is due to anti-inflammatory or antibacterial properties, or another unknown action, remains to be clarified. One case series reported that in 7 children treated with flucloxacillin, all had complete resolution, but only 4 children stayed in remission off therapy [43]. Additional antibiotics reported to effectively treat LABD in children are oxacillin, dicloxacillin, erythromycin and trimethoprim-sulfamethoxazole [36, 44–48].
Immunosuppressive Therapy
When patients with either prolonged disease who require further immunosuppressive therapy or with predominantly mucosal involvement need more aggressive therapy, steroid-sparing agents can be effective. In the authors’ estimate, these agents work in refractory disease. The order of preference is dictated by ease of use and severity of side effects [49–61].
Mycophenolate mofetil: This antimetabolite immunosuppressant is a prodrug of mycophenolic acid and is FDA-approved as an organ transplant rejection medication. It reversibly inhibits inosine-monophosphate-dehydrogenase, an enzyme in lymphocytes responsible for de novo guanosine nucleoside synthesis. Its use in dermatologic diseases, for which none are FDA-approved, has been increasing since its effective use in psoriasis. There are a few case reports of its successful use in cutaneous and oral LABD in children and adults [49, 50, 55, 56, 58].
Azathioprine: This corticosteroid-sparing immunosuppressant is a prodrug of 6-mercaptopurine, whose active metabolite is 6-thioguanine monophosphate. Its use in immunobullous disease is also not FDA-approved; however, its efficacy has been widely proven with numerous reviews on its use in this class of dermatologic diseases [62–64].
Cyclophosphamide: This alkylating agent is a nitrogen mustard derivative that directly damages DNA by cross-linking it, thereby inducing cell apoptosis. It is FDA-approved for mycosis fungoides; however, it is not approved for any immunobullous disease. This agent has shown particular efficacy when there is ocular involvement [65].
Intravenous Immunoglobulin (IVIg)
This non-immunosuppressive agent is pooled purified plasma from blood donors that contains supraphysiologic quantities of IgG and trace other immunoglobulins [66]. Its immunomodulatory effects are still not fully elucidated; however a few of its mechanisms of action include: suppression of the complement activation pathway through inhibiton of the membrane attack complex formation and the enzymes C3 and C5 convertase, neutralization of circulating autoantibodies, and blockade of the Fc receptors on macrophages and Fas ligand receptors on keratinocytes [67]. IgA levels must be checked before use of this medication as it can precipitate anaphylaxis in patients with IgA deficiency. IVIg has been successfully used alone and in combination with corticosteroids in patients with chronic renal failure and LABD, treatment-refractory LABD, and in those with chronic ocular involvement [52–54].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






