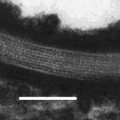
Fig. 21.1
Structure of lecithin
21.3 Physicochemical Properties of Lecithin Organogels
21.3.1 Viscoelasticity
The viscous and elastic nature of LOs follows the Maxwell model of viscoelasticity (Toshiyuki et al. 2003; Shikata et al. 2003). LOs are the three-dimensional structures formed as a result of physical interactions of lecithin molecules with each other. LOs show an elastic property at low shear rate. As the shear rate increases, the physical interactions among lecithin molecules start getting weak, and thereby the three-dimensional fibrous structure of LOs is distorted. Finally, the shear stress reaches its threshold and destroys the LOs’ structure. This behavior is termed as plastic flow behavior (Abdallah et al. 2000). The viscoelastic behavior of LOs depends on the concentration of lecithin and the type of organic solvent (precisely fatty acid ester) used. The higher the lecithin concentration, the higher is the viscosity (Schurtenberger et al. 1989). The long-chain fatty acid esters, such as isopropyl palmitate or cetearyl octanoate, produce LOs with high viscosity, whereas short-chain esters, such as ethyl and propyl acetate, produce LOs of relatively lesser viscosity. It is worthwhile to note that LOs can also be prepared by utilizing mixtures of solvents rather than using a single solvent. Using a blend of organic solvents, we can tailor the viscoelastic properties of LOs based on our needs. Nastruzzi et al. (1994) has developed LOs using a combination of organic solvents and investigated its effect on the viscoelastic properties of LOs. The viscosity of LOs has an impact on the release profile of the drug encapsulated into LOs. In general, the higher the viscosity, the slower is the release of the drug (Shchipunov and Shumilina 1995; Kumar and Katare 2005). Also, medicated LOs are reported to have slightly lesser viscosity than placebo LOs of similar composition (Shaikh et al. 2009; Nastruzzi and Gambari 1994).
21.3.2 Non-birefringence and Optical Transparency
The LOs when viewed under polarized light appear as a dark matrix. This is because the isotropic nature of organogels does not cause passage of polarized light through the matrix. This property of organogels is termed as non-birefringence (Kantaria et al. 1999; Nasseri et al. 2003). Optically, LOs are transparent and provide the benefit of visual inspection so that the presence of any particulate matter can be easily recognized (Kumar and Katare 2005).
21.3.3 Thermoreversibility/Thermostability
Heating above critical temperature causes LOs to convert from gel state to sol state (Fig. 21.2). The temperature at which sol-gel transition takes place is called as gelation temperature. The gelation temperature varies depending on the solvent system used when preparing LOs. The gel-sol transition in LOs occurs when increased thermal energy within LOs disrupts physical interactions between lecithin molecules, and subsequent cooling of hot LOs causes regain of physical interaction between lecithin molecules which results in sol-gel transition (Avramiotis et al. 2007). The gelation temperature can be determined visibly. It is essential to determine the gelation temperature of the LOs containing drugs as it serves as a guide for recommending storage conditions for drug-loaded medicated LOs (Díaz et al. 2008; Dasgupta et al. 2009; Guenet 2006).
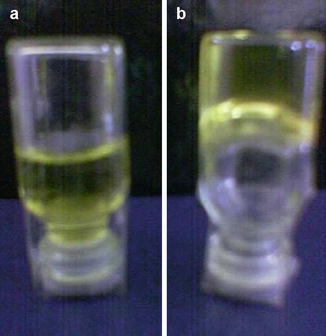

Fig. 21.2
Thermal behavior of the organogels. (a) Sol state of the LO at 60 °C and (b) semisolid state of the LO at room temperature
21.3.4 Biological, Physical, and Chemical Safety
Lecithin is an important component of all living cells, and it is recognized by the Food and Drug Administration (FDA) as Generally Regarded as Safe (GRAS) (21 CFR 184, 1400). Compatibility studies with human skin revealed that topical use of LOs is safe (Dreher et al. 1997; Shchipunov et al. 2001; Schurtenberger et al. 1990). Vehicles (organic solvents, such as fatty acid esters, e.g., isopropyl myristate, ethyl oleate) used for preparing LOs are also GRAS excipients. LOs are moisture insensitive, and due to their organic nature, they resist microbial contamination. Thermodynamic stability, ease of preparation and scale-up, easier quality monitoring, and enhanced topical performance along with biocompatibility and safety upon applications for a prolonged period make the organogels a vehicle of choice for dermal and transdermal drug delivery.
21.4 Salient Features of Lecithin Organogels (LOs)
LOs can encapsulate various substances with diverse physicochemical characters, i.e., having a different solubility, molecular weight, and size.
Self-assembled supramolecular arrangement of surfactant molecules imparts spontaneity to organogel formation and hence the process becomes simpler.
LOs remain structurally integrated for a longer period of time due to their thermodynamic stability.
LOs are not moisture sensitive; they also resist microbial contamination due to their organic nature.
Being well balanced in hydrophilic and lipophilic character, they efficiently partition into the skin and enhance the skin penetration and transport of molecules. LOs also provide the desired hydration of the skin in a lipid-enriched environment.
21.5 Preparation of Lecithin Organogels
The LOs are readily obtained by adding a minimal amount of polar solvent, such as water, to a solution of lecithin in organic solvents. They are formed by three different components, including organogellator (lecithin), a nonpolar organic solvent as external or continuous phase, and a polar agent, usually water. The transfer into jelly-like state has been demonstrated only for nonaqueous solutions of naturally occurring unsaturated lecithin (Walde et al. 1990; Shchipunov 2001).
21.5.1 Organogellator: Lecithin
Lecithin is a trivial name for 1, 2-diacyl-sn-3-phosphocholine. It belongs to a biologically essential class of substances termed phosphoglycerides or phospholipids. Lecithin is a complex mixture of acetone-insoluble phosphatides, which mainly consist of phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol combined with different amounts of other substances such as triglycerides and fatty acids (Reynolds 1996). The main sources of lecithin are soya beans and egg yolk. Lecithin varies greatly in its physical form, from viscous semiliquid to powder depending on the content of free fatty acids. It may also vary in color from brown to light yellow depending on whether it is bleached or unbleached (Wade et al. 1994). Lecithin is commercially available on the market under trade name of Epikuron (Lucas Meyer, Hamburg, Germany), Lipoid S100 (Lipoid GmbH, Ludwigshafen, Germany), and Capcithin (Lucas Meyer, Hamburg, Germany), which are derived and purified from either soya bean or eggs. The desired gelation in organic solvent occurs only when the lecithin contains more than 95 % phosphatidylcholine and is free from fat as well as moisture. Lecithin is a multifunctional surface-active agent. The lecithin molecule consists of two portions: the nonpolar tail (fatty acid portion) and the polar head (phosphoric acid portion). Because of these two aspects, lecithin molecules arrange themselves at the boundary between immiscible liquids such as oil and water. This arrangement reduces the interfacial tension between oil and water and makes relatively stable emulsions (Scartazzini et al. 1988). Its unique lipid molecular structure performs versatile functions. It has a wide variety of roles in pharmaceuticals, cosmetics, and food industries as an emulsifier, viscosity modifier, stabilizer, and solubilizer and penetration enhancer (Szuhaj 1989). They form the lipid matrix of biological membrane and play a key role in the cellular metabolism (Hanahan 1997). Due to its biocompatibility, it is widely used in human and animal food, medicine, cosmetics, and other various industrial applications (Wendel 1995). No systematic research has been done till date in order to investigate the effect of unsaturation in phospholipids on organogelling ability. However, it has been reported that unsaturation in phospholipid molecules affect the nature of self-assembly in which the phospholipid molecules associate and form the microstructures. The property of unsaturation can be interpreted in terms of the degree of hydration of phospholipid molecules that it provides. Unlike saturated hydrogenated phospholipids, unsaturation in phospholipid molecules would result in better hydration of the polar head group, thereby increasing the area per lipid polar head group. Hence, a larger area-to-volume ratio would favorably alter the spontaneous curvature of lipid monomers for the formation of micelles and subsequently their self-assembly to form the micellar network (Shchipunov 2001).
21.5.2 Organic Solvents
Organic solvent plays a vital role in organogels by providing the desired solvent action for the drug (hydrophobic) as well as for lecithin (Kumar and Katare 2005; Moore 1982; Sato et al. 1988). More than 50 organic solvents have been reported to form organogels with water as an aqueous phase. Among them, there are linear, branched, and cyclic alkanes; ethers and esters; fatty acids; and amines. Specific examples include ethyl laureate, ethyl myristate, isopropyl palmitate, cyclopentane, cyclooctane, trans-decalin, trans- pinane, n-pentane, n-hexane, n-hexadecane, and tripropylamine. Among these, the fatty acid esters have been widely used for LO formation (due to better skin feel), but structural investigations have only been performed on hydrocarbons (such as isooctane, cyclohexane). Natural oils including soya bean oil, sunflower oil, rapeseed oil, and mustard oil are proposed as potentially useful organic solvents for preparing LOs (Shchipunov 2001).
21.5.3 Polar Solvent
The third component, a polar agent, acts as a structure forming and stabilizing agent. A series of polar solvents have been studied in order to check their suitability for producing the thickening effect on hydrocarbon and fatty acid ester solutions of lecithin. Water has been used extensively as a polar solvent for organogel formation. However, it has been established that glycerol, formamide, and ethylene glycol have the ability to induce gelation. The gel-forming ability of the polar solvent is governed by its physicochemical properties (Shchipunov and Shumilina 1995, 1996; Shchipunov and Hoffmann 1998). The ability to promote thickening of lecithin solutions has been correlated with the polarity of the solvent used for preparing LOs. This correlation is particularly pronounced in the series of structurally related solvents such as glycerol and ethylene glycol. In a proposed model of organogels, the solvent molecules bridge phosphate groups of neighboring lipid molecules, allowing their association into tubular aggregates through an extensive ribbonlike hydrogen bonding network (Shchipunov 2001). Figure 21.3 depicts schematic diagram of the preparation of LOs.
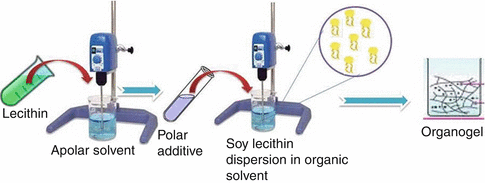

Fig. 21.3
Schematic diagram of the preparation of LOs
It is important to understand that LOs cannot be formed by random mixing of lecithin, an organic solvent and polar solvent. It depends on the mixing of a fixed quantity of these three components. Selection of suitable amounts of these three components can be done by making use of the pseudo ternary phase diagram. A detailed description on how to estimate the determination of the required amount of lecithin/water/organic solvent is described in the next section.
21.6 Identification of Appropriate Quantities of Lecithin/Organic Solvent/Water Using Phase Diagram
The phase diagram provides information for the boundaries of the different phases as a function of composition variables. The phase behavior of a ternary system of lecithin/organic solvent/polar solvent is mainly governed by the concentration of polar solvent and lecithin (Shchipunov 2001; Shchipunov and Schmiedel 1996). It is defined in terms of a parameter, molar ratio of polar solvent to lecithin (nw = [polar solvent]/[lecithin]). When the polar solvent is water, nw is also termed w o (w o = [water]/[lecithin]). Nw is the critical molar ratio of polar solvent to lecithin molecules where gel formation and entrapment of external organic phase occur.
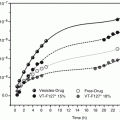
For LOs prepared using different organic solvents but fixed lecithin concentration, the nw value varies from solvent to solvent. For isopropyl myristate-based LOs with a lecithin concentration of 200 mM, nw value is reported to be 3, whereas for ethyl myristate-based LOs with a lecithin concentration of 200 mM, nw value is reported as 5 (Nastruzzi and Gambari 1994). LOs exist in a narrow characteristic range of water-to-lecithin molar ratio, which is reported as ncr. It is the critical molar ratio of polar solvent to lecithin molecules at which complete gel formation takes place and entrapment of maximum amount of external organic phase is observed. When nw is equal to ncr, LOs with maximum viscosity are produced, and when nw exceeds ncr, phase separation of the LOs occurs (Shchipunov 2001; Shchipunov and Schmiedel 1996a, b). The rheological measurements or visual and optical observations of a ternary system between cross polarizers are widely used to determine ncr values. For a better understanding on the construction of a phase diagram, we wish to describe an example from our reported paper on LO-containing aceclofenac (Shaikh et al. 2009).
A pseudo ternary phase diagram was constructed to determine the concentration range of lecithin, organic solvent (ethyl oleate), and water required for aceclofenac containing LO. This study was carried out for the lecithin concentration ranging from 10 to 60 % w/v because lecithin in proportion above 60 % w/v could not be solubilized. Initially, water in oil microemulsion was stabilized by lecithin micelles formed with a low concentration of water, which is characterized by optical transparency and low viscosity (Fig. 21.4a). As the amount of water was increased, the microemulsion turned to a viscous gel (Fig. 21.4b). Further increase in water resulted in turbidity appearance (Fig. 21.4c) and finally phase separation (Fig. 21.4d). Organogel existence area was obtained from the above pseudo ternary phase diagram (Fig. 21.5).

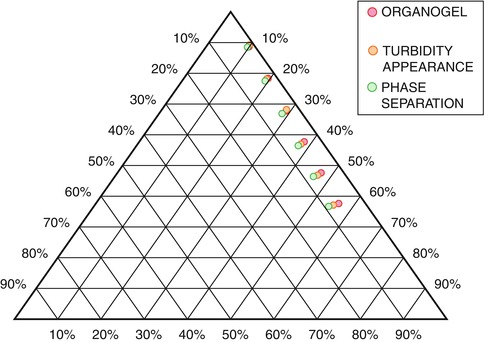

Fig. 21.4
Changes in lecithin-/ethyl oleate-based system with addition of water. (a) Water in oil microemulsion with low viscosity and optical transparency, (b) microemulsion turned into viscous transparent gel at wo = 4, (c) turbidity appearance wo > 4, and (d) phase separation at wo > 5

Fig. 21.5
Ternary phase diagram for ethyl oleate/lecithin/water system
Based on the outcome of the phase diagram experiment, it was inferred that the occurrence of the gel phase was lecithin concentration dependent. The water-holding capacity of lecithin organogel increases with the increase in lecithin concentration. Lecithin concentrations ranging from 10 % to 60 % w/v were found to be optimum for preparing LOs.
21.7 Mechanism of Formation Lecithin Organogels (LOs)
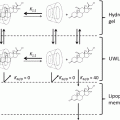
The first prerequisite for gel formation is the balance of intermolecular interaction among the gelator molecules (e.g., H bonding, van der Waals interactions, etc.) and between gelator and solvent molecules. A comparative increase in the intermolecular attraction among the gelator molecules and a comparative decrease in the interaction between the gelator molecules and solvent lead to the formation of a molecular dispersion, which further results in the formation of a three-dimensional network in which the solvent molecules are trapped (Fig. 21.6).
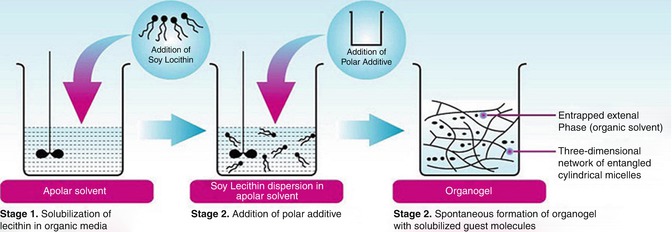

Fig. 21.6
Entanglements of cylindrical reverse micelles to form gel
Lecithin forms reverse spherical micelles spontaneously when dissolved in apolar solvent alone at a concentration of ~0.01 mM (Shchipunov et al. 1998). The enormous uniaxial growth of these spherical reverse micelles and subsequent transformation into tubular or cylindrical micellar aggregates (sphere-to-cylinder transformation) is triggered by the addition of small and critical amounts of polar additive as shown in Fig. 21.7.
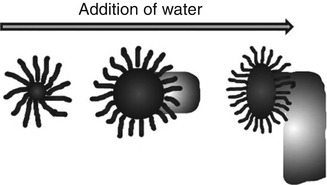

Fig. 21.7




Transformation of spherical micelles into cylindrical micelles
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree