Fig. 15.1
Operating room arrangement and port placement
Trocar placement is a highly variable and yet critical step toward a safe and successful operation. Most authors describe external landmarks such as the umbilicus or xyphoid to determine placement. However, obese patients have a high degree of abdominal wall thickness with corresponding varying degrees of rigidity. Also, the size of the liver and presence of previous operations and their associated internal adhesions will determine initial and subsequent trocar placement. One must recognize that placement of the trocars needs to accommodate manipulation and construction of both the small bowel and hiatus, often challenging with the larger patients. Therefore, we feel it better to place the trocars based on internal anatomy, rather than external landmarks. In this way, triangulation and visualization will be preserved, accommodating for variations in the size of the liver or length of the patient’s torso.
Attention must be given not only to individual trocar placement but also to the angle in which the trocar enters the skin. Some individuals’ thick, muscular abdominal wall does not allow for the range of motion necessary to achieve the objective, forcing redirection of the trocar internally, through the same skin incision, but different fascial opening, or by placement of another trocar. In general, the optimal placement is to orient all trocars toward the midline, pointing to the base of the mesocolon.
Extra long trocars may be necessary. Although some surgeons prefer to limit the number of 12 mm trocars (necessary to accommodate stapling devices), this may limit proper stapler orientation and compromise the anatomic construct. The hernia risk is minimized by either closing the trocar defects or, preferably, using non-bladed trocars without fascial closure.
An example of a trocar placement scheme is as follows: its purpose is only to illustrate the rationale necessary for consistency of this important and underappreciated first step in performing the laparoscopic gastric bypass. Variations, depending on experience, technique, and judgment, are necessary for evolution to occur and should be encouraged:
1.
Initial trocar (12 mm)—left, upper quadrant, subcostal, and midclavicular line. This is often an optical entry without prior insufflation. The rationale is that many patients have had previous procedures, pelvic or otherwise—this area is rarely affected with intra-abdominal adhesions from common open procedures. This allows dissection of midline adhesions, inspection of the size of the liver, and determination of the best level for the primary optical port. This will also be the primary port for vertical stapling of the gastric pouch. Once adhesions are mobilized, then the optical port can be thoughtfully placed as to see the ligament of Treitz as well as hiatus without having to “turn around.” Also, by keeping the initial entry away from the midline, the vena cava and aorta are not as vulnerable to injury.
2.
Primary optical trocar (12 mm)—placement has been described above. Optimal placement allows for forward visualization of the proximal small bowel and the hiatus. Once this trocar is placed, the camera is moved to this port for subsequent trocar placement. I have not found the current 5 mm scopes to provide enough light delivery and therefore resolution for optimal visualization in most patients.
3.
Right-sided trocar (12 mm)—this trocar must be placed thoughtfully just as all others. Exterior landmarks are irrelevant. It must come in below the liver edge, just to the right of the midline so as to be able to triangulate on the hiatus as well as the ligament of Treitz; therefore, it should be angled toward the root of the mesocolon, rather than perpendicular to the abdominal wall. It must be 12 mm to accommodate the stapler that will define the inferior gastric pouch.
4.
Left inferior trocar (12 mm)—this is often at the same level as the primary optical trocar and in the same line as the initial trocar. This will be the primary stapler entry site for the jejunojejunostomy (Fig. 15.2) and along with the right upper quadrant trocar will triangulate very well for a comfortable manual gastrojejunostomy (Fig. 15.3).
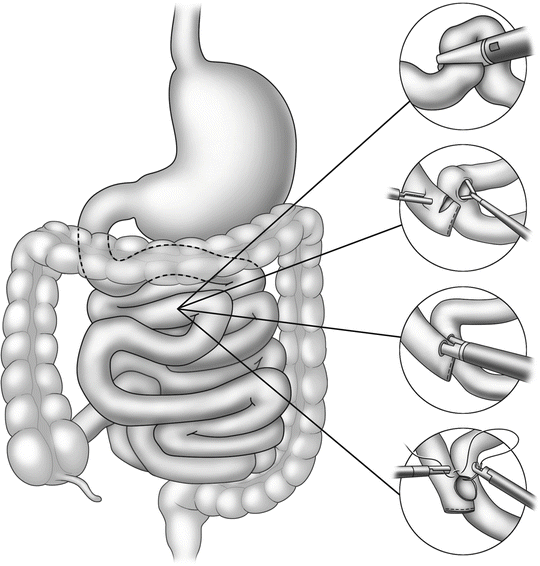
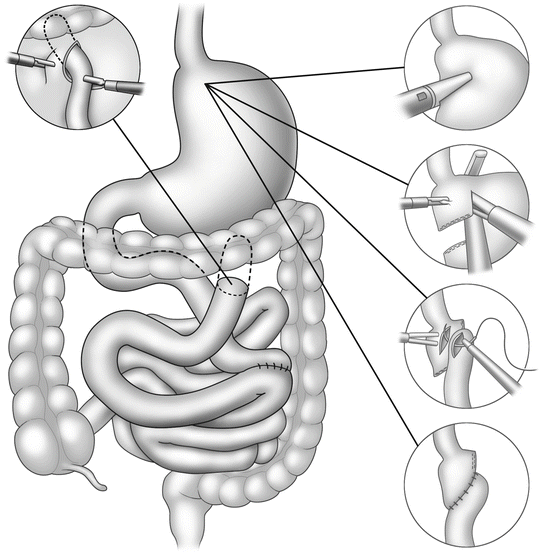

Fig. 15.2
Steps in jejunojejunostomy

Fig. 15.3
Steps in gastrojejunostomy
5.
Liver retractor—the most consistent placement appears to be subxiphoid. A 5 mm trocar can be used here, depending on the liver retractor of choice. We have found that a simple 5 mm instrument or similar device will provide excellent exposure and therefore is often placed without a trocar, through direct puncture, as it will not be removed until the end of the case.
The Components
The Pouch
Much emphasis is placed on the formation of the gastric pouch or modification of the gastric pouch to improve performance. Its contribution to the “gastric bypass” effect is undeniable, yet poorly understood. Collateral evidence suggests that the actual size and configuration of the pouch are more important for prevention of complications and improved alimentation rather than maximal weight loss or metabolic effect. In other words, one would be hard-pressed to find literature, anecdotal or otherwise, that describes a linear correlation between pouch size and weight loss. Likewise, attempts at pouch reduction for weight recidivism or inadequate weight loss have been inconsistent and disappointing.
Early gastric bypass descriptions were of horizontal orientations and often nondivided staple lines. Both have been retired with the adoption of linear cutting stapling devices allowing for orientation and construct based on intent, rather than necessity. One can now orient the pouch vertically with complete isolation of the gastric pouch with more precision and less concern for splenic or other visceral injury. This was made even easier with the laparoscopic stapling devices; along with the increased visualization of the proximal stomach and hiatus, the laparoscopic approach has improved the safety and reproducibility of this procedure.
Isolation of the gastric pouch was important to limit the potential of gastrogastric fistula—relatively common complication of nondivided staple lines. It also allowed for more precise dissection—nondivided pouches were often created by placement of a spherical intragastric balloon for sizing, rather than a cylindrical bougie. This made the pouch consist primarily of anterior stomach wall with a greater amount of fundus that accounted for its eventual dilation and increased capacity. Vertical orientation of the pouch has been shown by Mason to be ideal for gastroplasty, avoiding the distensible fundus, making the pouch more stable in size, an observation.
The pouch is formed by sequential firing of a laparoscopic linear cutter, stapling device around an intraluminal bougie. The first firing is usually horizontal beginning no more than 5 cm distal to the esophagogastric junction; subsequent firings are vertically oriented to the angle of His. The staple height depends upon the thickness of the tissue, often requiring 3.8–4.1 mm cartridges. The size of the bougie does not appear to be controversial; most surgeons use the same size to calibrate the gastrojejunal anastomosis—usually 1.2–1.5 mm diameter.
One of the critical steps in creation of the gastric pouch is posterior visualization at the level of the hiatus. Many surgeons will “bluntly” dissect behind the stomach, but given the variable level of adhesions to the pancreas and the splenic vessels, this is unwise. Optimally, it is better to enter the lesser sack through the gastrocolic omentum and free the posterior gastric adhesions up to the esophageal hiatus. This protects the pancreas and the occasional tortuous splenic artery from inadvertent injury. After this, the lesser curve, perigastric dissection can be performed with more confidence and placement of the stapler more precisely so as not to “twist” the stomach pouch. This occurs when posterior gastric adhesions prevent the initial horizontal stapler from capturing equal amounts of anterior and posterior gastric wall. The resultant twist is not as critical as in the gastric sleeve but looks funny nonetheless.
More controversial is whether or not to dissect the hiatus and repair a hiatal hernia when present. Autopsy studies show that a hiatal hernia is present in up to 70 % of individuals, similar to our observations when we routinely dissect out the hiatus in all patients. However, dissection of the hiatus can add additional time and potential complications to an already complicated procedure. Our studies have not shown that preoperative endoscopy accurately predicts the absence of a hiatal hernia; the only way to determine its presence is circumferential dissection of the esophagus. The absence of the “anterior” dimple is not reliable as the hernia space is often taken up by a large paraesophageal lipoma that can be easily reduced into the abdomen once identified. Once identified, the hiatal hernia is best repaired posteriorly.
The question remains: “Is it important to repair every hiatal hernia at the time of gastric bypass?” The answer is not clear. If one assumes that precise dissection and formation of the gastric pouch are important to limit postoperative complications, then it would be appropriate to absolutely identify the location of the gastroesophageal junction—often hidden in a “sea of fat”—to better perform more consistent reconstruction. It has been our observation that almost 100 % of patients we have reoperated after gastric bypass have a significant hiatal hernia at the time of reoperation—something not appreciated at the time of the first intervention.
Dissection of the hiatus and repair of the hiatal hernia along with removing the fat pad overlying the angle of His may allow for more precise and consistent pouch formation and subsequent better long-term performance and lower complications, but this has not been proven. Although this may add up to 5–10 min of operative time, which is significant, the added exposure may make for a safer operation. Each surgeon will need to evaluate this perception in the context of his or her individual experience and skill level. Suffice it to say that to perform a good laparoscopic gastric bypass, the surgeon must be expert at hiatal dissection.
The Bypass
The vertical banded gastroplasty has not been shown to be as effective as the gastric bypass in terms of long-term weight loss and quality of life owing to significant GERD and persistence of appetite and hunger. Clearly, the intestinal bypass is an integral part of the physiology of the gastric bypass even though the mechanism of its contribution is still largely unknown.
Overall intestinal length can vary as much as 100 %, and intraoperative measurements are far from precise given the dynamism of the small bowel. Still, attempts to accurately measure and modify gastric bypass limb lengths to correlate with weight loss and malnutrition have been published. Very few comparative studies exist; most show no difference except in the super obese population and then only for a few years. Our data suggests no difference between a 100 and 150 cm Roux limb when stratified to patients with a BMI greater or lower than 50 kg/m2. Varying biliopancreatic limb lengths up to 100 cm likewise have failed to show a difference. Therefore, it does not seem critical or necessary to expose gastric bypass patients to excessive diversion beyond that which is necessary to prevent bile reflux to the gastric pouch.
Extreme variants of the proximal gastric bypass that include radically lengthening the Roux limb, shortening the common channel to 50–150 cm from the ileocecal valve, or shortening the entire alimentary limb length to 3–4 m, effectively creating a biliopancreatic-type diversion, do not constitute what is commonly referred to by patients and insurers as “gastric bypass.”
The length of the biliopancreatic limb is not critical, usually just long enough to provide mobilization of the Roux limb to the gastric pouch without tension. This depends on whether or not the Roux limb is to be routed antecolic or retrocolic, antecolic naturally requiring more length. The length of the Roux limb should be at least 75 cm as we have seen bile reflux in patients with 60 cm Roux limbs. Variants up to 150 cm give no added benefits. Beyond 150 cm, insufficient data exists to illuminate any benefit.
The bowel is transected with a linear cutting stapling device. Surprisingly, little mesentery division needs to be performed; one must be careful not to transect the superior mesenteric artery.
Construction of the jejunojejunostomy is usually performed as a side-to-side anastomosis with linear cutter staplers (Fig. 15.2). Attention must be directed to avoid kinking or twisting the Roux limb or inadvertently performing the notorious Roux-en-O by not properly identifying each limb prior to anastomosis.
The Anastomosis
The least controversial but most often studied component of the laparoscopic gastric bypass is the gastrojejunal anastomosis. Three distinct methods exist. The original method proposed by Wittgrove and Clark involved endoscopic retrieval of a percutaneous guidewire that was then attached to the anvil of a 21 mm circular stapler. This allowed the anvil to be passed orally, into the gastric pouch; the stapler was passed through an enlarged port site through an opening of the Roux limb so as to create the anastomosis. After retrieval, the afferent limb was then transected. The anastomosis can then be inspected and tested with the flexible endoscope. Care must be taken when the anvil passes the cricopharyngeus—the narrowest part of the esophagus.
Scott and de la Torre modified placement of the anvil through a gastrotomy prior to gastric pouch formation, eliminating the need for operative endoscopy or transoral passage of the anvil [5]. The gastrotomy required closure similar to the enterotomy made on the Roux limb with the Wittgrove method.
Other surgeons used the linear cutter stapler to create a side-to-side anastomosis from the Roux limb to the gastric pouch [6]. This technique required manual closure or the residual opening, but minimal suturing was required.
The simplest but overlooked method of creating this anastomosis is a handsewn approach familiar to most open bariatric surgeons. It is unclear why many minimally invasive surgeons dismiss this technique as being too difficult when much of the operation still requires manual suturing skill. The handsewn anastomosis remains the most cost-effective method of gastrojejunostomy. It remains the surgeon’s choice at to the suture material, single or two layer and interrupted or continuous, as well as the diameter of the thread and size of the needle. We prefer 3-0 absorbable sutures for this application.
Routing of the Roux Limb and Closure of Mesenteric Defects
The Roux limb can be brought through the mesocolon (retrocolic) or anterior to the colon (antecolic) as well as anterior or posterior to the gastric remnant. While all routes are acceptable, one must be familiar with all methods so as to be able to adapt to any situation. For example, if one has planned, or the situation requires, a gastrostomy tube, then a retro-gastric placement of the Roux limb will allow the gastric remnant to attach, unimpeded, to the abdominal wall.
The antecolic routing eliminates one potential site of herniation—the mesocolon—but introduces additional tension on the gastrojejunal anastomosis by the weight of the colon and undivided omentum, if present. When necessary, the omentum can be shifted to the right of the patient or divided; under no circumstance is a trans-omental route acceptable for the potential for herniation through the omental defect and possible small bowel obstruction. The large Petersen’s space should be closed either way.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






