CHAPTER 47 Jumbo Cups
Acetabular revision with cementless components has been remarkably successful, with some series reporting no revisions for aseptic loosening at an average follow-up of 13.9 years.1 Cementless acetabular revision is now feasible for a wide range of revision situations, including some cases of pelvic discontinuity. The Paprosky classification is useful in predicting the reconstructive technique that will be required (see Table 33-3). Type I and many type II defects may be reconstructed with standard cementless components. A standard cup may be ineffective with increasing bone loss.
Many type II and type III defects, which involve the loss of additional structural bone, can be reconstructed with a jumbo cup. A jumbo cup is defined by Whaley and colleagues as a component that is >61 mm in women and >65 mm in men, a definition that is based on a shell that is >10 mm greater than the diameter of the average cup implanted in women and men.2
Jumbo acetabular components can be used in combination with both structural and cancellous bone graft. In these cases the cementless cup must achieve adequate contact with host bone in order to allow bone ingrowth to occur. The percentage of host bone contact that is considered adequate is not known exactly, but a recent study suggests that 50% contact with host bone is desirable.3
INDICATIONS AND CONTRAINDICATIONS
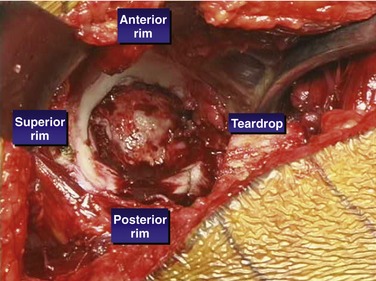
Jumbo cups are indicated for revision of type II and III defects in which stable two-point fixation can be achieved. The two points of fixation can be obtained between the anterior and posterior walls, between the anterosuperior and posteroinferior acetabulum, or between the posterosuperior and anteroinferior acetabulum (Fig. 47-1). The initial stability of the cup should be adequate before the use of screws for supplemental fixation. The cup can be used for segmental, cavitary, and combined defects of the acetabulum. There must be adequate bone to provide initial support for the cup, and the cup must have minimal micromotion in the healing phase for bony ingrowth to occur. Also, there must be adequate bony contact and bone quality to allow biologic ingrowth to occur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree