10 Pancreatic islet transplantation
Introduction
Diabetes mellitus (DM) comprises a group of common metabolic disorders that share the phenotype of hyperglycaemia and is caused by progressive loss of insulin secretion or action. The metabolic dysregulation associated with DM causes secondary pathophysiological changes in multiple organs. Insulin is produced by β cells within the islets of Langerhans. It is estimated that in the adult human there are approximately 1 million islets scattered through the pancreas.1 DM affects 15.7 million individuals in the USA, is the leading cause of end-stage renal disease, non-traumatic lower extremity amputations and adult blindness, and contributes $92–138 billion directly to healthcare costs and an additional $45 billion in lost productivity.2 The incidence of DM is increasing in the USA and worldwide, and 30 000 new cases are diagnosed each year in the USA.3 As such, diabetes represents a target disease that will have tremendous impact on society in terms of raising the quality of life and freeing healthcare resources for other diseases.
The principal determinant of the risk of devastating diabetes complications is the time of exposure to hyperglycaemia. In this regard, the therapeutic use of insulin significantly improved the survival of patients with type I diabetes by preventing acute lethal complications like diabetic ketoacidosis.4 Improved patient survival, however, allowed the development of secondary complications. Several studies demonstrated that the incidence of these complications can be reduced by tight glycaemic control but the risk of severe hypoglycaemic reactions is then increased.5,6 The only approach at present to restore sustained normoglycaemia is endocrine replacement therapy. In this regard, transplantation of the whole pancreas (normally simultaneously with a kidney transplant) is now an established treatment option for some type I diabetics. But this is only an option for a small group of diabetic patients with advanced disease, and even in the most experienced hands it has a high morbidity and modest mortality (40% and 3–5% respectively).7 The pancreatic islets only account for around 1% of the volume of the pancreas and so transplantation of the exocrine pancreatic tissue is unnecessary and carries added risk.
 Transplantation of islets alone offers an attractive alternative to whole pancreas transplantation and may be associated with a lower incidence of serious complications.8
Transplantation of islets alone offers an attractive alternative to whole pancreas transplantation and may be associated with a lower incidence of serious complications.8
The first islet transplant was attempted in 1893 in Bristol – 28 years before the discovery of insulin – whereby fragments of a sheep’s pancreas were implanted in the subcutaneous tissues of a 15-year-old boy dying from uncontrolled ketoacidosis.9 This xenograft was destined to fail without immunosuppression. The era of experimental islet research began in 1911, when Bensley stained islets within the guinea-pig pancreas using a number of dyes and was able to pick free the occasional islet for morphological study.10 Mass isolation of large numbers of viable islets from the human pancreas has proven to be a challenge ever since. The techniques used today for the mass isolation of human islets evolved from earlier techniques used to prepare islets in rodent models.11
Islet transplantation for type I diabetes has been attempted in many units over the last 10–15 years with poor long-term success. Of over 400 islet transplants reported to the International Transplant Registry between 1990 and 2000, less than 10% resulted in 1-year insulin independence.12 These transplants were carried out on a heterogeneous group of diabetic patients and, in the majority of cases, included glucocorticoids in the immunosuppression protocol.
 A report published by the Edmonton group of seven consecutive type I diabetic patients who all attained long-term insulin independence after islet transplantation renewed interest in islet transplantation as a feasible option for a select group of diabetic patients.13 These patients were all transplanted using a glucocorticoid-free immunosuppressive regimen, immunogenic xenoproteins were eliminated from islet preparations and high islet numbers, mostly from two pancreases, were used to achieve insulin independence.
A report published by the Edmonton group of seven consecutive type I diabetic patients who all attained long-term insulin independence after islet transplantation renewed interest in islet transplantation as a feasible option for a select group of diabetic patients.13 These patients were all transplanted using a glucocorticoid-free immunosuppressive regimen, immunogenic xenoproteins were eliminated from islet preparations and high islet numbers, mostly from two pancreases, were used to achieve insulin independence.
Islet transplant activities
Islet transplantation has been conducted mostly in non-uraemic type I diabetic recipients as islet transplants alone. However, patients can be considered for islet transplantation while immunosuppressed for another organ transplant, usually kidney. The results of combined islet and kidney transplantation now match those of islet alone14 and recent data suggest that islets transplanted with a kidney may prolong the patient and kidney graft survival and protect against diabetic vascular complications.15,16 In addition, islets can be infused in combination with other abdominal organs (islets and liver or islets in combination with liver and small bowel).17 Autologous islet transplantation has been successfully executed in patients subjected to total pancreatectomy for chronic pancreatitits or trauma.18 Few cases of islet xenotransplantion (pig to human) have been reported.19
Patient selection for islet transplantation
Inclusion criteria for islet transplantation are outlined in Box 10.1. The major indications for islet transplantation are hypoglycaemic unawareness and metabolic lability. Assessment of these complications is subjective and a number of scoring systems have been devised to allow quantification of these problems. The mean amplitude of glycaemic excursion (MAGE) using 14 blood glucose values over a 2-day period has previously been used in the assessment of potential recipients;20,21 however, it has been superseded recently by the combination of a lability index (LI) and composite HYPO score based on 4 weeks of glucose values.22 The HYPO scoring system takes into account the frequency, severity and degree of unawareness of hypoglycaemia, and has not been shown to be significantly higher in islet transplant patients pretransplant. It becomes normal post-islet transplant. Ryan et al. suggested that the HYPO score provides a more objective assessment of the metabolic instability of an individual patient and allows pre- and post-transplant comparison. Patients must be assessed by an endocrinologist and to benefit from transplant they should have continuing problems despite an optimum insulin regimen. Ultimately, the decision whether to offer islet transplantation depends on the individual patient and should be arrived at by balancing the risks of the islet transplant procedure itself and potentially lifelong immunosuppression against the daily risks taken by a patient with type I diabetes. Current recipient exclusion criteria for solitary islet transplantation are listed in Box 10.2.
Box 10.1 Indications for islet-alone transplantation
(Despite ‘state-of-the-art’ insulin therapy defined by monitoring of blood glucose values no less than four times each day and by the administration of three or more insulin injections each day)
Box 10.2 Exclusion criteria for islet-alone transplantation
Human islet processing
Regulatory aspects
Despite considerable clinical success, islet transplantation is still considered an investigational procedure in most countries. Therefore, it is regulated to safeguard public health and monitor the development of new products.23 In the USA, islet grafts are regulated by the Food and Drug Administration (FDA) as biological products, and an Investigational New Drug (IND) application must be submitted and approved by the FDA before the initiation of any studies in humans subjected to islet transplantation. Pancreas processing for islet isolation must be performed in purpose-built Clean Room Facilities with strict adherence to current Good Manufacturing Practices (cGMP).24
Pancreas procurement for islet isolation
An ideal donor for human islet recovery will have a favourable medical, sexual and social history, pass the physical examination requirements, and clear all standard laboratory tests used in multiorgan donor work-ups to show low risk of disease transmission. Pancreases are generally obtained from deceased, heart-beating organ donors (DBD), but successful isolations have been reported from non-heart-beating donors and living hemipancreas donors.25,26 The surgical technique for removal of the pancreas for islets should be the same as that for solid pancreas transplantation, taking care to avoid direct handling of the pancreas and keeping the capsule intact. The pancreas can be removed before or after the liver, or en bloc with the liver and separated on the back table.27 Care should be taken to keep the pancreas cold after cross-clamping and during back-table preparation/packaging of the organ, as this has been shown to double the yield of viable islets.28 Ryan et al. have shown that the ischaemia index, which is the cold ischaemic time for a given transplanted islet mass, correlates with insulin secretion.29 Ideally, the cold ischaemia time should not exceed 8–12 hours. Donor factors associated with successful isolations include body mass index (BMI) >25 kg/m2, age (lower islet recovery in donors <18 years old), adequate donor glycaemic control before organ procurement, and local procurement team.30,31
Islet isolation
 The currently recommended, semi-automated process for human islet isolation was described by Ricordi et al. and is based on combined collagenase digestion and mechanical dissociation of the donor pancreas.32
The currently recommended, semi-automated process for human islet isolation was described by Ricordi et al. and is based on combined collagenase digestion and mechanical dissociation of the donor pancreas.32
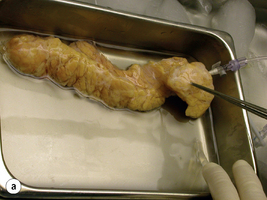
First, the pancreas is briefly exposed to an antibiotic and antifungal solution. Extrapancreatic fat and non-pancreatic tissue is removed and the pancreatic duct is cannulated. The pancreas is then perfused under controlled conditions with collagenase (Fig. 10.1a). For several years, the most consistent results were obtained using a purified enzyme blend (Liberase HI, Roche), which has more consistent collagenase activity combined with neutral protease activity and low endotoxin levels;33 however, newer enzyme blends such as Collagenase NB1 (Serva) are now the only available product for clinical islet recovery for transplantation. Following distension, the pancreas is transferred to the Ricordi digestion chamber and circuit (Fig. 10.1b,c). Here the temperature of the collagenase is raised to 37 °C to allow activation of the enzyme and digestion of the pancreas. Samples are taken at regular intervals to monitor, via inverted microscope, the breakdown of the pancreas. The digestion can be stopped when the intact islets are free from the surrounding exocrine tissue. This part of the isolation process requires skills and experience and is critical to the whole process. The pancreatic digest is purified to separate the islets from other products of digestion such as ductal tissue, lymphoid tissue and necrotic cells with purification gradients (ficoll or iodixanol) and a Cobe 2991 cell separator.34 Transplantation of unpurified digests can result in insulin independence; however, this approach increases the risk of portal vein thrombosis, portal hypertension and disseminated intravascular coagulation (DIC).35
The resulting purified islet preparation is then washed, counted and assayed to make sure that it meets the product release criteria of the isolation facility. These criteria are essential to ensure that a safe, pure and effective islet preparation is consistently produced by the isolation facility (Box 10.3). Safety is measured by negative Gram stain and endotoxin assay and islet purity (ideally >50%) determined by eye, although computerised counting software is currently under evaluation. The likely potency of an islet preparation is difficult to determine prior to transplantation and several investigations are under way to develop reliable assays of islet potency and function. Current practice is to use a combination of islet counts and cell viability stains such as fluorescein diacetate/propidium iodide and SytoGreen/ethidium bromide to determine the viable β-cell mass.13 Islet function is quantified by glucose-stimulated insulin release assays.13 Newer techniques, such as islet oxygen consumption rate, fractional β-cell viability, ADP/ATP content, and quantification of basal and stimulated reactive oxygen species (ROS) levels showed good correlation between product testing and in vivo islet function in animal studies and may be useful in the near future.36–40 Islet graft characterisation by histological analysis has also shown good correlation with post-transplant outcomes.41