Trial (year)
Local recurrences %
Follow-up (years)
Breast-conserving surgery
Breast-conserving therapy
NSABP B06 (2002)
39.2
14.3
> 20
Milan III (2001)
23.5
5.8
10
Swedish (1999)
24
8.5
10
British (1996)
35
13
5
Scottish (1996)
24.5
5.8
5.7
Table 14.1b
Survival: role of radiotherapy according to the biggest trial
Trial (year) | Survival % | Follow-up (years) | |
|---|---|---|---|
Breast-conserving survey | Breast conserving therapy | ||
NSABP B06 (2002) | 46 | 46 | > 20 |
Milan III (2001) | 76.9 | 82.4 | 10 |
Swedish (1999) | 78 | 77.5 | 10 |
British (1996) | – | – | 5 |
Scottish (1996) | – | – | 5.7 |
New studies were undertaken in an attempt to identify subgroups of patients, tumor characteristics, and surgical techniques that might achieve adequate local control using breast conservation without irradiation [1, 2]. Surgery alone has failed to obtain local control as effectively as that seen with surgery followed by WB irradiation in trials with long-term follow-up. The latest Early Breast Cancer Trialists Collaborative Group (EBCTCG) systematic review confirmed a 75% reduction in local recurrence (LR) risk after radiotherapy, and showed that the prevention of four local recurrences prevents one cancer-related death at 10 years, corresponding with 1–5 fewer deaths per 100 node-negative patients and 5–10 fewer deaths per 100 node-positive patients treated [3].
Subsequently the local-regional management of BC has arrived at another crossroad. The standard radiotherapy is being replaced, in several clinical trials, by shorter courses of postoperative irradiation lasting only 1–5 days or intraoperative irradiation (“accelerated”), and focused solely on the breast tissue around the surgical cavity (“partial breast”).
If APBI is demonstrated as safe and effective in selected women compared to WBRT in ongoing trials, this will represent a new option for selected women with early-stage BC.
14.2 APBI: Theoretical Bases
14.2.1 Whole-breast Radiotherapy Criticisms
The daily visits that women must make to a treatment facility over 5 weeks are disruptive, especially in these situations:
Women with family or work obligations
Elderly women
Women with important comorbidity, and
Distance from the facility, especially in case of a lack of reliable transportation [4].
In these situations, an important percentage of patients must choose between avoiding postoperative radiotherapy (undertreatment) or deciding for a mastectomy (overtreatment). Another topic, especially in a period of economical crisis, is represented by consumption of resources: breast irradiation may constitute 25– 30% of patients for a radiation oncology service and it can create stress for a health-care delivery system.
In addition, APBI provides a better integration with chemotherapy for women who require such treatment. Finally, it has been demonstrated that external boost may often miss the target (simply because it is more difficult for the radiotherapist to know the exact tumor site than for the surgeon during the operation).
14.2.2 “Topographical Distribution” of Tumor Recurrences
After BCS, most breast cancer recurrences are localized at the site of the original tumor rather than elsewhere in the breast [5–6]. Accordingly, the WBRT approach is potentially unnecessary and may introduce avoidable toxicity.
BCT trials have demonstrated that the majority of in-breast tumor recurrences take place near the original tumor site in or around the surgical site, within 2 cm (known as “true recurrences”) and those recurrences which develop away from the initial tumor (“elsewhere failures,” or “new or second, ipsilateral primary breast cancer”) may not be significantly reduced by radiotherapy. 70–86% of the local recurrences after BCS without radiotherapy are true recurrences, according to the trials reported in Table 14.1 (Milan III: 85%; NSABP B06: 70%; Swedish group: 72.7%)
14.2.3 Radiobiological, Clinical, and Psychological Aspects
During or immediately after surgery, the tissue is maximally vascularized and oxygenated, since radiotherapy acts with a mechanism of ionization and free radical liberation that causes DNA damage, the effect in the case of APBI is optimized. In addition, DNA damage can be repaired between each delivery while this cannot happen in the case of single-step irradiation.
Using a mathematical model known as the linear-quadratic equation, it has been concluded that shorter plans require more intense doses of radiation per fraction to achieve the same effect on the tissue. In addition, higher doses are better tolerated as the target volume decreases.
The therapeutic effect seen with a standard course of 50 Gy to the whole breast at 2 Gy per day (plus a boost to the tumor bed) might be seen equally well with 21 Gy in a single fraction using intraoperative radiotherapy (IORT). The dose is delivered to the target under direct vision. The adjacent tissues are spared: they can be easily shielded or moved away from the radiation field, finding a solution to the problem of cardiac and lung exposure and the related sequelae. Moreover, skin and subcutaneous tissue are also spared, with possible improvement of cosmesis [7].
It is obviously better for a woman to complete the treatment (surgery and radiation therapy) at the same time or in a short period instead of in many weeks [8].
14.3 Techniques
There are some possibilities of performing an APBI approach.
14.3.1 Interstitial Brachytherapy
Studies with longer follow-up started with this approach, in the mid 1990s by Frank Vicini. The rates of local recurrence in the APBI arm versus the standard arm have been demonstrated to be similar, equally disease free survival (DFS) and overall survival (OS).
Up to 20 catheters are inserted in the breast tissue surrounding the tumor cavity under direct control during surgery. Radioactive sources (High Dose Rate (HDR) unit or Low Dose Rate (LDR) iridium seeds) are loaded afterward in the catheters to irradiate the cavity plus a margin. For HDR, a schedule with 34 Gy in 10 fractions (twice daily) over 5 days is the most frequently used. At the end of treatment, the catheters are removed.
14.3.2 MammoSite Balloon Catheter Using HDR Brachytherapy
An inflatable balloon linked to a single- or multi-lumen catheter is inserted into the surgical cavity, during or after surgery, under ultrasound guidance. The balloon is inflated and the source (usually iridium) is inserted. The balloon-to-skin distance should be 5–7 mm: a shorter distance may lead to poorer cosmetic results or skin necrosis. For this reason, it is not indicated for patients with small breasts or superficial tumor. It is important to make the walls of the cavity adhere to the device and then inject contrast for radiographic verification of the correct positioning, with respect to the target and to the structures that are to be spared. This technique respects the heart and lungs, but can also potentially lead to fat necrosis within the breast. The most frequently used schedule is 34 Gy in 10 fractions (twice daily) over 5 days. Harper published data that revealed acceptable toxicity and comforting cosmetic results [11, 12].
14.3.3 External Beam Radiation with 3D-Conformal Radiation Therapy (3D-CRT) and Intensity-modulated Radiation Therapy (IMRT).
APBI can also be performed with the new generation linear accelerators of that are already present in most RT departments. These methods require external beams but, with the use of an accurate study of sagittal, coronal and transverse planes, it is possible to reconstruct with extreme precision the target volumes excluding important anatomical structures. With sophisticated software even synchronization with the respiratory movements can be taken into consideration. The most frequently used schedule is 38.5 Gy in 10 fractions (twice daily) over 5 days [10, 13].
14.3.4 Intraoperative Radiotherapy Using the Photon Radiosurgery System (TARGIT)
Intraoperative radiotherapy using the photon radiosurgery system (TARGIT) uses low-energy x-rays directed through an applicator sphere (intrabeam) placed in the surgical cavity with breast tissue sutured around it. The tumor bed, with no supplementary margin, is irradiated to a dose of 20 Gy. This technique requires dedicated equipment, operating room time and technical expertise. Critical points are the low penetrability, the difficulty (for the steric dimensions) of a correct positioning and an adequate distance from the skin. Treatment schedule: two doses per day for 5/7 days [14].
14.3.5 Intraoperative Radiation Therapy Using Electrons (IORT)
Intraoperative radiation therapy using electrons (IORT) is used to deliver a single fraction (one-step treatment) in the operating room during BCS. A mobile linear accelerator producing 3–10 MeV electron beams is used in combination with an electron applicator placed over the surgical cavity, delivering a single fraction of 21 Gy to the tumor bed plus a margin of 1.5–3 cm. Like TARGIT, it requires dedicated equipment, operating room time and technical expertise. Compared to other APBIs, IORT with electrons offers the most homogeneous dose distribution, with an average dose inside the target volume closest to the prescribed dose [7, 15]. In addition, IORT, as the other all-in-one approaches, avoids any delay in local and systemic treatments. Frozen section analysis is clearly the most important weakness of intraoperative technique, as the definitive pathology may reveal contraindications to a limited radiation field [7]. For more details see Section 14.6.
14.4 APBI: Selection of Patients
The APBI treatment currently cannot be considered as the gold-standard approach [7]. The standard is still represented by the external postoperative treatment. Up to now, there is not enough evidence to perform APBI as the first choice in any patients: it should be undertaken within clinical trials, after obtaining the informed consent of the patient, or in out-trials but in a selected group of patients. Thus, the take home message must be: “select patients with great care”. Obviously those subjects with high risk-factors for LR must be excluded.
14.4.1 General Elgibility Criteria [16–26]
The eligibility criteria are summarized in Table 14.2. Before and during the operation, it is important to assess the absence of an extensive intraductal component (EIC; defined as 25% or more of the area encompassed by the infiltrating tumor) that is considered to be an important risk factor for LR in breast-conserving therapy.
Table 14.2
APBI: criteria for the selection of the eligible patients
Age and hormonal status | Post menopausal status, 48–75 years: age has a primary role in the development of local recurrences. The incidence is greater in pre-menopausal women where the risk of occult multicentricity of the tumor is higher, due to different anatomy/biology of breast gland |
Histology | Invasive carcinoma (in situ carcinoma is often multifocal/ multicentric). Ductal better than lobular carcinoma (more likely multifocal) |
Foci | Unifocality |
Dimensions (T) | Tumor diameter ≤ 25 mm: the bigger the size, the higher the risk of other foci of tumor |
Axillary status | No significant involvement of the axilla present: in such a case it will be necessary to perform radiotherapy on the lymphatic regional stations |
Radiotherapy contraindications | No factors contraindicating RT in general (i.e., a previous treatment already done in the same site) |
The margins involvement is the other factor to evaluate before starting the treatment. It is well known that the presence of margins with disease significantly reduces the effectiveness of radiation therapy in general and of the partial treatment in particular, in terms of prevention of LR.
For these reasons, it is mandatory to have a good collaboration with the pathologists in a multidisciplinary approach in order to assess the margin-status as well as possible: some trials require an intraoperative evaluation of the margins and in the case of positive/close margins a postoperative standard treatment is indicated. The most important disadvantage of all intraoperative techniques is that a definitive histology of margins is not available at the time of treatment.
Oncoplastic techniques, performed after APBI, can significantly improve cosmetic outcome and they allow the surgeon to perform removal with wide margins that are more likely to be negative.
Because of the difficulty of obtaining a secure evaluation of the margins during the operation, it is essential to perform an accurate preoperative assessment of the disease and the type of breast. For this purpose, a magnetic resonance of the breast is particularly useful in order to exclude, with good approximation, multifocality (22%) and/or contralateral breast involvement (5%): its use results in a change of surgical approach in approximately 15% of patients (Fig. 14.1) [22–24]. However magnetic resonance cannot exclude with absolute certainty the presence of peritumoral disease (which means positive margins). In this sense, a selection of patients is mandatory to avoid doubtful cases.
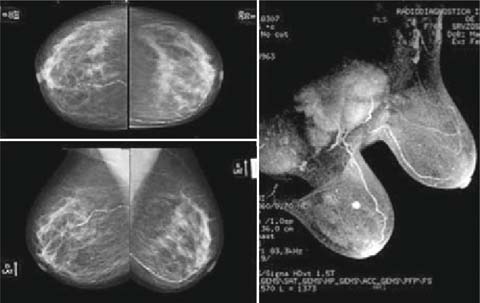

Fig. 14.1
Breast MR is particularly useful in order to exclude multifocality, especially in the case of a dense breast
American ASTRO and European GEC-ESTRO have published guidelines analyzing three categories of patients [25, 26]:
1.
Low-risk of local recurrence. APBI suitable, also acceptable outside a clinical trial; including patients > 50 or 60 years, with unicentric, unifocal, lesions < 3 cm, nonlobular invasive breast cancer without EIC and lymphovascular invasion (LVI), with negative surgical margins (> 2 mm) and without axillary node involvement
2.
Intermediate-risk or cautionary group, for whom APBI is considered acceptable only in the context of prospective trials
3.
High-risk or unsuitable group, for whom APBI is considered contraindicated; patients 40–50 years of age or younger, with involved margins, and/or multi-centric or large tumors, and/or presence of EIC or LVI, and/or >three positive lymph nodes or unknown axillary status.
Other factors may have a significant relation with local recurrence, even if they are not part of the guidelines, such as HER2 amplification, proliferation index, biological subtype (basal cell vs. luminal A type).
14.5 IORT
There are different experiences, most advanced of which (in terms of follow-up and enrollment sample size) is the study ELIOT at IEO, Milan [27, 28]. In Italy, there have also been other experiences, including ours in Pisa (as part of a multicenter national trial) that started in 2003.
A mobile linear accelerator with a robotic arm is used to deliver electron beams able to produce energies from 3 to 9 MeV. Through a perspex applicator (collimator) of 4–10 cm diameters (usually 5–7 cm, 0° angle), radiation is delivered directly to the mammary gland.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






