Fig. 13.1
Valley of Death. Drug and device development sometimes comes to a halt if additional funds cannot be secured for the leap from the preclinical to the clinical research phase. This may happen for a variety of reasons, most typically lack of investor confidence in the potential to recoup development costs as well as a reasonable return on investment
Investigator $150–200 million
CRO $50–100 million
Central labs $10–15 million
Monitors $8–12 million
Longer approval times encroach on revenues. Typical patents grant 20 years of exclusivity, but 12–15 years are lost to clinical trials and other research and development. A typical NDA requires 70 studies; 91,000 pages of regulatory documents, and costs $359 million. Documentation for the regulatory process (IND, NDA) alone is about 3 % of R&D costs, but still amounts to $24 million. Delays in approval cost $684,000–$1 million/day. Total estimated cost of bringing new drug to market is $800 million–$1.4 billion. These are Tufts CSDD data from 2010. Other sources such as Public Citizen estimate the costs to be much lower, on the order of $110 million overall. Public Citizen attributes the discrepancy to the following:
Many drugs receive federal support for development.
Drug companies deduct 34 % for R&D.
Tufts CSDD figures include opportunity costs.
Nevertheless, regardless of methodology, cost of research is going up because study subjects are more complex, new study medications and devices are more complex, protocols are more complex, and regulatory requirements are more burdensome. Recent studies have also confirmed that the burdens of healthcare research and development costs are tilting away from the NIH and toward industry (Fig. 13.2).
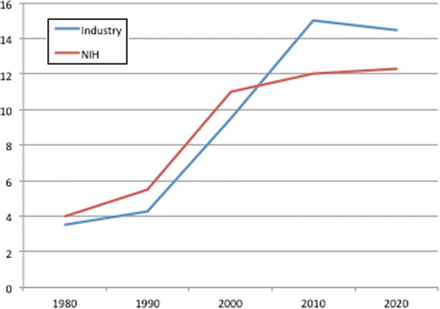

Fig. 13.2
The proportion of pharmaceutical research being funded by industry is increasing and supplanting but not completely offsetting the declines in funding from the NIH
Inset 13.1
Some studies take decades to come to fruition. The study of phosphodiesterase in inflammatory mediator began in the 1950s. Subsequently, the role of phosphodiesterase inhibitors began to be understood for inflammatory and autoimmune diseases. More specific inhibitors such as apremilast were developed in the past decade and have progressed from pilot studies to multi-center Phase III trials for psoriasis.
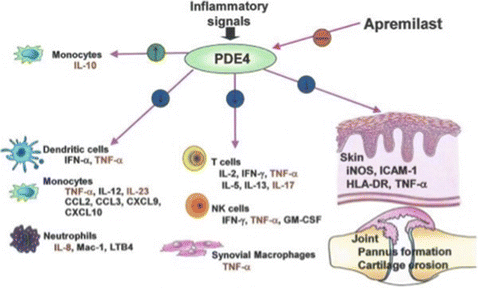
Kumar N, Goldminz AM, Kim N, Gottlieb AB. Phosphodiesterase 4-targetedtreatments for autoimmune diseases. BMC Med. 2013;11:96.
An open-label, single-arm pilot study in patients with severe plaque-type psoriasis treated with an oral anti-inflammatory agent, apremilast. Gottlieb AB, Strober B, Krueger JG, Rohane P, Zeldis JB, Hu CC, Kipnis C. Curr Med Res Opin. 2008;24(5):1529–38.
Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, Hu C, Day RM. Lancet. 2012;380(9843):738–46.
Now two Phase III Trials: ESTEEM 1, ESTEEM 2. http://clinicaltrials.gov/show/NCT01194219, http://clinicaltrials.gov/show/NCT01232283.
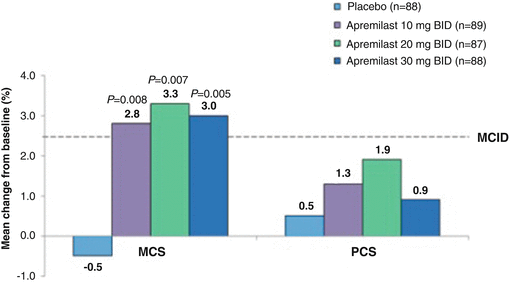
Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements inpatient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health Qual Life Outcomes. 2013;11:82.
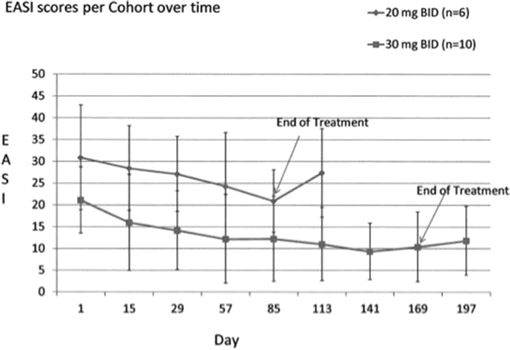
Apremilast has also recently been studied for atopic dermatitis. As an example of a proof-of-concept pilot study, which employs surrogate biomarkers as well as subjective improvement scores, quality of life measures, and objective clinical measures to track response, apremilast was used tested in adults. An investigator-initiated open-label pilot demonstrated small-scale proof-of-concept efficacy on a group of 16 adults with atopic dermatitis. They were treated with apremilast 20 mg or 30 mg twice daily and evaluated for (1) adverse events, and (2) for improvements in pruritus, DLQI, and EASI. Significant reductions were noted in all three measures as well as gene-based measures.
Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148(8):890–7.
13.1.1 Costs of Clinical Trials
In the early mid-1970s, the cost to pharmaceutical companies of developing a drug was $100 million adjusted for inflation. Thirty years later, costs had escalated to $1.3 billion. By 2011, the costs had gone up to nearly $5.8 billion per drug.
The principal driver of cost has been the regulatory process of Phase III studies of human subjects (Fig. 13.3). Over the past decade, Phase III trials have involved more subjects and become more complex than previously. The number of procedures of clinical trials (laboratory evaluation, imaging, examinations) has increased by 70 %. There has been a commensurate increase in the burden on investigators, clinical trial staff at the investigative site and staff associated with the sponsor and trial research or management organization, leading to more staff required and more work hours required of staff members. This is even true for dermatologic studies. For example, studies of psoriasis drugs were limited to topical agents. But systemic agents such as retinoids or biologic agents such as tumor necrosis factor inhibitors carry greater risk and require extensive laboratory evaluation. Simple examination of psoriasis subjects has been replaced by detailed PASI scores, NAPSI scores, standardized photography of psoriatic lesions, and quality of life assessments such as the DQLI. The length of clinical trials has increased by 70 %. This may be due to the complexity of the trials themselves, or to longer follow-up to observe persistence of a positive effect or the development of adverse events. Trial sizes may be larger to demonstrate a more subtle effect or to ferret out rarer side effects. Complex protocols with stringent enrollment criteria mean longer enrollment periods and higher dropout rates. This has led to a 20 % drop in enrollment rates compared to earlier trials. Because of the added burden of complex trials on subjects, the retention rate of volunteers has also dropped (by about 30 %).
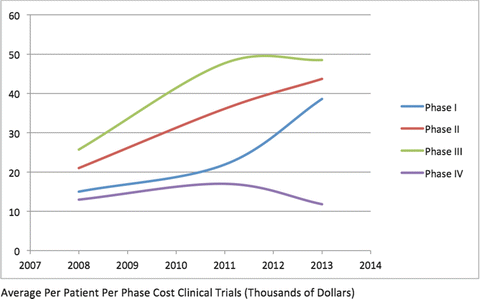

Fig. 13.3
The costliest arm of clinical drug development is Phase III
Current estimates suggest that about 40 % of a company’s total Research & Development expenditures go toward Phase III clinical trials. This may be an underestimate, because the overwhelming majority of new molecular entities never make it to Phase III of development. If an analysis on just those entities which are approved is done, 90 % or more of the total cost of development (from bench to bedside) is in Phase III. Furthermore, only one in 12 drugs which enter into Phase III clinical trials receive final FDA approval.
Added costs have several consequences in the industry. Small companies have difficulty sustaining the costs of trials to completion, especially if there is a glitch or setback in the study process. Investors are nervous about supporting pharmaceutical enterprises, and are quick to withdraw funds for the slightest reason (delays in clinical trial milestones, emergence of competitors, delays in FDA approval). Otherwise promising drugs and devices wither in the development phases. The result is higher healthcare costs and a dearth of potential therapies, and reduced overall quality/cost ratio of health care.
Regulatory agencies require that new drug or device application show significant evidence that a drug or device is beneficial. This should be done through well-controlled trials conducted by qualified experts. At least two studies are required, meaning two large-scale, multiyear Phase III clinical trials. These must show a benefit with 95 % statistical certainty (p < 0.05).
Industry is given regulatory relief in the management of rare or orphan diseases. In the case of orphan drugs, smaller and less costly Phase III clinical trials are permitted. For example, for a medication to treat paroxysmal nocturnal hemoglobinuria, approval required only 184 patients for Phase III, and 206 patients overall Phase I–III. Even for rare disease drugs, 90 % or more of the development costs are in Phase III. Some oncology drugs are given expedited approval after a successful Phase II trial.
The result is that many drugs and devices with potential benefit are not reaching patients. For example, Arena pharmaceuticals developed a drug for the treatment of obesity which was effective compared to placebo and did not demonstrate any significant side effects. Regulators did not approve the application because manufacturers were unable to show that the drug did not cause heart valve disease. Denied approval, and forced to prove a negative, the company’s stock price plummeted, and its research has to go back to the drawing board. Two other antiobesity drugs were rejected that year (2011), one because the company could not prove that the drug didn’t increase a subject’s risk of heart attack. In effect, three proven to be effective antiobesity drugs, with trials enrolling 18,000 subjects were summarily taken down by regulators. Drugs which had the potential to reduce diabetes, heart disease, osteoarthritis, as well as a host of other ailments, including dermatologic ones, were halted, representing a tremendous setback for the management of obesity, an epidemic. Congress acted to require the FDA to take steps to support new treatments for obesity. The agency, after convening an independent panel of experts, is now reconsidering the drugs, but may require further studies costing hundreds of millions of dollars. Narrow disease-by-disease legislative action is rare and cumbersome.
Risk aversion is not just limited to regulators. Investors may pull the plug on a molecular entity in the early phases of development even if it shows promise. The reasons may vary: concern about competitors, concern about the numbers needed to show an effect, and concern about regulatory delays or denials based on the current state of the agency. This means that some products never reach the bedside.
Industry would like to see changes in the regulatory model, which it sees as outdated. Studies in the past were for acute illnesses, such as infectious diseases, where treatment results were dramatic and could be measured rather quickly. More and more current research is targeting chronic illnesses such as diabetes, hypertension, dementia, stroke, and cancer. In dermatology, treatments are aimed at managing chronic diseases such as adult acne, atopic dermatitis, psoriasis, and skin cancer. It may take years to measure beneficial effects for these conditions.
The approval process is also binary. A drug or device is approved for all its indications, all patients, and can be marketed through all prescribed channels. But if it is not effective for even one subset of the application, it is withheld from patients.
Costs of trials are high, and this cost is inherent in the current regulatory environment. Regulatory agencies have a high standard of proof, and broad authority to decide what makes a trial acceptable or not. Regulatory agencies can always ask more questions, and suggest further studies, in effect moving the bar at any point in the study process, even after Phase III trials are over. This leaves developers with a lingering sense of uncertainty over the future prospects of their discovery.
One remedy that industry seeks is an end to black/white, yes/no binary approval and a more graduated conditional or incremental approval. In this scenario, regulators could grant limited approval to drugs and devices after successful Phase II trials. The selection of patients eligible for medication would be narrow depending on the data. The income generated from early sales could be used to fund Phase III trials for final broader approval.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







