Cause of failure
Presentation
Associated factors
Recurrent instability
Early (<6 months)
Poor operative technique
Failure of graft incorporation
Premature return to high-demand activities
Overly aggressive rehabilitation
Late (>6 months)
Repeat trauma to the graft
Poor graft placement
Concomitant pathology not addressed
Generalized ligament laxity
Complications
Stiffness
Global arthrofibrosis
Poor preoperative range of motion
Prolonged postoperative immobilization
Intercondylar notch scarring
Cyclops lesion
Nonanatomical graft placement
Graft overtensioning
Complex regional pain syndrome
Infection
Surgical contamination
Multiple procedures
Comorbidities
Extensor mechanism dysfunction
Quadriceps muscle inhibition
Loss of patellar mobility
Inadequate rehabilitation
Joint-related pain and arthritis
Chondral defects
Postmeniscectomy pain
Recurrent Instability
The primary goal of an ACL reconstruction is to restore the anterior and rotational stability of the knee following ACL injury. Continued or recurrent instability of the knee following an ACL reconstruction is most likely due to a deficient graft and is universally considered a surgical failure. The incidence of ACL graft failure and recurrent instability has been reported between 0.7 and 10 % of primary ACL reconstructions [11–14]. This scenario is the primary indication for a revision ACL reconstruction.
The mechanism of graft failure can be broken down into three categories: technical errors during primary procedure, insufficient biologic healing, and traumatic reinjury (Fig. 6.1). Most studies have identified technical errors as the most common etiology of graft failure, causing more than 50 % of the failures [5, 7, 15]. However, most of these studies identified one primary cause for failure and neglected any contributions from the other categories. ACL graft failure is a multifactorial process, with a significant interrelationship from each component. Recently, the MARS (Multicenter ACL Revision Study) group published initial results on their first 159 patients enrolled in this multicenter, prospective cohort evaluating failure modes in revision surgery. The study found isolated traumatic injuries to be the biggest indication, producing 32 % of the revision cases, followed by technical errors in 24 % of patients. Multiple etiologies were identified in 35 % of patients, with 55 % of cases describing some contribution of trauma to failure, and 53 % having a technical error component to their graft (Fig. 6.1) [16].
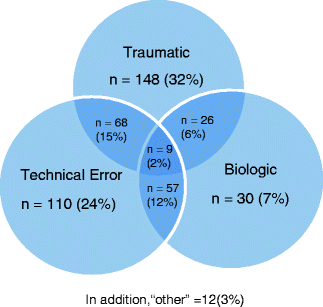
Table 6.2
Nonanatomic positioning of bone tunnels
Tunnel | Position | Results |
|---|---|---|
Femoral | Anterior | Excessive strain (i.e., lengthening) in flexion/laxity in extension |
Posterior | Excessive strain in extension/laxity in flexion | |
Central/Vertical | Rotational instability | |
Tibial | Anterior | Excessive strain in flexion/roof impingement in extension |
Posterior | Excessive strain in extension/impingement vs. PCL | |
Medial | Impinges on medial femoral condyle/impingement vs. PCL | |
Lateral | Impingement on lateral femoral condyle |

Fig. 6.1
Causes of ACL failure (from Wright RW, Huston LJ, Spindler KP, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010; 38:1979–1986. ©American Journal of Sports Medicine; Reprinted by permission of SAGE Publications)
In addition to the MARS study, more recent studies are finding some history of trauma in most failed ACL grafts [17], with incidences between 24 and 100 % of revision ACL cases [7]. However, whether the traumatic event was the true initiator of the instability, or rather the result of an already deficient graft first noticed by the patient is sometimes difficult to ascertain. Injuries to the ACL graft can present early in the postoperative course, within 6 months of the primary procedure, or as a late presentation outside of 6 months. Although some patients may describe a traumatic event, reasons for early failure include poor surgical technique, delayed graft incorporation [14], loss of graft fixation [18], premature return to high-demand activities [19], and overly aggressive rehabilitation [20].
Traumatic rerupture after 6 months requires a force similar in magnitude to cause an original ACL tear [15]. With appropriate surgical technique and rehabilitation, long-term prospective cohorts have found the risk of graft ruptures to be the same as injuring the contralateral, “normal” knee with an incidence between 5 and 10 % at a minimum 5-year follow-up [17, 21, 22]. In fact, one systematic review found that the risk of ACL tear in the contralateral knee was double (11.8 %) the risk of ACL graft rupture (5.8 %) [23]. Risk factors for traumatic reinjury include surgical errors, young age, and participating in competitive pivoting, jumping, or contact sports. With increasing expectations of young athletes to return to high-level sports after ACL reconstruction, the number of traumatic reinjuries after ACL reconstruction is expected to increase.
Technical errors in ACL reconstruction include malpositioned tunnels, inadequate notchplasty, improper tensioning, and insufficient graft fixation. More than 70 % of technical errors are due to malpositioned tunnels [16, 24], with anterior femoral tunnel positioning the most common error. Positioning of the femoral tunnel is extremely important to knee function, as the origin of the ACL is close to the axis of rotation of the knee [8]. Small changes in the femoral attachment of the ACL have significant effects on the knee’s biomechanics. Poor tunnel placement leads to excessive changes in graft length over the knee’s range of motion, leading to plastic deformation and consequently loosening of the graft. An anterior femoral tunnel places increased stress on the graft in flexion, resulting in decreased flexion, pain with flexion, and stretching of the graft [15]. Similarly, a femoral tunnel too posterior increases the stress on the graft in extension, resulting in loss of extension, pain on extension, and stretching of the graft as well. Over time, both scenarios could result in deformation of the graft to the point of incompetency.
The femoral tunnel can also be incorrectly positioned in the coronal plane. A tunnel too close to the central axis of the femur, at the so-called 12 o’clock position, will result in adequate anterior restraint but poor rotational stability [25, 26]. Failure to control for rotation will result in continued instability episodes with cutting or pivoting activities. The femoral tunnel needs to be positioned more horizontally on the medial wall of the lateral femoral condyle to best control for anterior and rotation stability [27].
The effects of tibial tunnel positioning are more forgiving on graft tension than femoral tunnel positioning, but also play a role with potential impingement of the graft. Similar to femoral tunnel positioning, an anterior tibial tunnel will result in increased graft tension in flexion, whereas a posterior tibial tunnel will result in decreased tension in extension. In addition, an anterior tibial tunnel will cause impingement of the graft against the notch in extension, resulting in pain and/or decreased extension [28]. A posterior tibial tunnel will impinge against the PCL in flexion, causing pain and/or decreased flexion. Medial or lateral placement of the tibial tunnel may also cause impingement against the medial and lateral walls of the intercondylar notch [29]. The tibial tunnel position is not the only factor in graft impingement. Typically, the ACL graft is larger than the native ACL, so adequate space may not be available within the intercondylar notch. Even with ideal tibial tunnel position, a notchplasty may be required in some knees to open up the space available for the incoming graft to prevent impingement from occurring [30, 31]. Prevention of impingement is important, as repetitive friction from knee range of motion may cause persistent pain and swelling, as well as ultimately lead to graft failure and knee instability.
In addition to tunnel position, multiple other factors are involved in graft tension, including preoperative laxity, graft type, fixation type, and knee flexion angle at time of fixation [2]. Several randomized clinical trials have attempted to evaluate the effect of intraoperative graft tension on clinical outcomes after ACL reconstruction [32–35], but currently the optimum graft tension is unknown. Grafts that are undertensioned at the time of fixation are too loose, resulting in more knee instability postoperatively. Grafts with too much tension may result in decreased vascularization and delayed graft incorporation, myxoid degeneration, decreased graft strength, and overconstraining of the knee [36], which may potentially lead to osteoarthritis in the long term.
With recent emphasis on advanced rehabilitation protocols stressing early range of motion, it is imperative that graft fixation techniques be able to maintain graft tunnel position and tension until biologic incorporation has occurred [18]. Aperture fixation with interference screws gives the best fixation biomechanically [18, 37]. However, complications associated with their use include improper sizing of the bone plugs, osteopenic bone, divergent screw placement relative to the bone plug, and transection of the graft [18, 37–39]. With the increasing use of soft-tissue grafts, more suspensory types of fixation are being utilized with their own unique problems. Fixation points are farther from the joint, resulting in increased graft length, decreased stiffness, and increased displacement during cyclic loading [40]. The bungee cord effect and windshield-wiper effect described motion of the graft within the tunnel which can cause tunnel widening, loss of graft tension, and loss of graft fixation. Finally, with the increasing number of fixation devices available, proper surgical technique is important to avoid errors that may adversely affect graft fixation.
During an ACL reconstruction, secondary structures of the knee must also be evaluated and treated for a successful outcome. In a study of 80 ACL reconstructions, all patients who had postoperative clinical instability with giving way demonstrated evidence of associated ligamentous instability that had not been appreciated or addressed at the time of the primary surgery. Kamath et al. report that 3–31 % of ACL failures were due to missed collateral instability or concomitant malalignment [7], while Getelman and Friedman identified 15 % of revision ACL cases due to failure to address associated knee laxities [41]. Structures that need to be evaluated include the posterior horn of the medial meniscus, the posteromedial and posterolateral corners, and the overall alignment of the lower leg. The medial meniscus acts as an important secondary restraint to tibial translation, and increased forces are noted in the reconstructed ACL in the meniscus deficient knee [19]. Unrecognized injuries of the posterolateral or posteromedial structures result in unnatural high forces seen in the ACL graft as well, which result in gradual attenuation and eventual early failure [42]. Varus malalignment, either solitary or combined with medial compartment narrowing from complete or partial meniscectomy, may result in varus thrust in the limb, leading to repeated stretching and fatigue on the reconstructed ACL [43]. If identified early enough, these concomitant pathologies can be treated before the ACL graft becomes incompetent. However, once these injuries go unrecognized after the primary procedure, they are generally not discovered until the ACL graft has failed and revision ACL reconstruction is required.
Biologic failure of the ACL graft is failure of incorporation and ligamentization [44], resulting in an atonic, disorganized, and nonviable graft. This mode of failure should be considered in a patient with recurrent instability without a history of trauma and with no detectable technical errors, including injuries to secondary stabilizers. Graft incorporation involves a sequential, regulated process of necrosis, revascularization, cell repopulation, collagen deposition, and finally matrix remodeling [19]. Failure of this process is due to avascularity, immunologic reaction, and stress shielding. Still, very little is known about the biologic variables that affect the rate and extent of ACL graft incorporation. Several mechanical factors influence the vascularity to the graft and subsequent graft incorporation, such as intercondylar notch impingement, graft overtensioning, postoperative immobilization, infection, and immunologic reactions [37, 45, 46]. Thus, biologic healing of the graft can be optimized with appropriate surgical technique and postoperative rehabilitation.
Stiffness
Early studies of ACL reconstruction identified stiffness as the most common complication postoperatively, with high rates ranging from 24 to 35 % [47, 48]. Etiologies for stiffness include preoperative swelling or stiffness, infection, poor compliance with physical therapy, reflex sympathetic dystrophy, prolonged immobilization, impingement, scarring or capsulitis, and poor surgical technique. With the reduction of risk factors and accelerated rehabilitation protocols, stiffness as a postoperative complication has markedly decreased to as low as 0–4 % of cases [49–52]. Such modalities include ensuring full range of motion preoperatively, as well as immediate range of motion, immediate weight bearing, early quadriceps exercises, and patellar mobilization postoperatively. Surgical intervention is occasionally required should rehabilitation fail and includes manipulation under anesthesia, arthroscopic lysis of adhesions, or open lysis of adhesions [47, 48]. As mentioned previously, a malpositioned bony tunnel can result in stiffness and pain. If loss of range of motion and an intact, sometime tight, ACL graft are found in combination with tunnel misplacement, arthroscopic graft resection and arthrolysis may need to be considered if all other modalities have failed. Following surgical treatment, the knee should be treated with intensive physical therapy to regain full range of motion, and then consideration for revision ACL reconstruction, should the patient complain of knee instability.
Arthritic Pain
One of the elusive goals of an ACL reconstruction is to prevent or delay the development of osteoarthritis. However, multiple factors are postulated to be involved in the development of osteoarthritis after an acute knee injury. The initial hemarthrosis following a traumatic knee injury and the associated inflammatory response may initiate the arthritic pathway early on in the knee [53–55]. The associated structural damage from the injury, including bone bruises, articular cartilage damage, and meniscal pathology, may also affect the development of arthritis in the knee. Finally, recurrent episodes of instability that occur from the time of injury until the ACL reconstruction may result in further damage predisposing the knee to arthritis.
Several long-term, prospective studies have evaluated the effect of articular cartilage and meniscal injuries on outcomes following an ACL reconstruction. Ichiba and Kishimoto found lower knee patient-reported scores and higher osteoarthritis scores in patients with meniscal tears or articular cartilage damage following an ACL reconstruction [56]. Shelbourne and Gray demonstrated an inverse relationship between patient-reported knee scores and amount of meniscus removed during a meniscectomy [57]. Their group also recognized worse outcomes in patients with articular cartilage damage. Finally, Wu et al. found more subjective complaints, lower scores, lower performance on objective testing, and more arthritic changes on radiographs with patients who underwent meniscectomies compared to patients with intact menisci following their ACL reconstruction [58]. Thus, a successful ACL reconstruction with a stable knee may have an unsuccessful outcome because of the initial traumatic event or other associated pathologies. When evaluating a patient for revision ACL reconstruction, it is important to differentiate pain due to arthritis, meniscal, or articular cartilage injury from pain due to instability.
Extensor Mechanism Dysfunction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








