Fig. 43.1
Patient in group I (“ex-obese”). Immediate preoperative/abdominoplasty marking by the technique of Costa et al. [26]
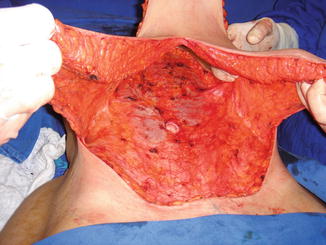
Fig. 43.2
Patient in group I (“ex-obese”). Detachment of cutaneous flap above the anterior abdominal aponeurosis
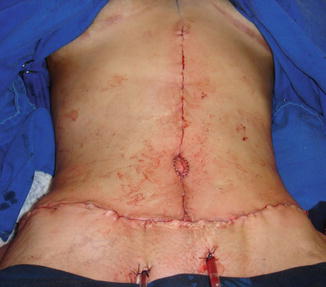
Fig. 43.3
Patient in group I (“ex-obese”). End of surgery with scar “anchor” and subcutaneous drain output with the drain on pubis
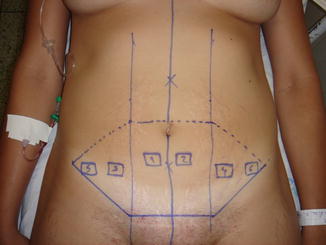
Fig. 43.4
Patient in group II (“control/nonobese”). Immediate preoperative/marking for abdominoplasty by Pitanguy technique [33]
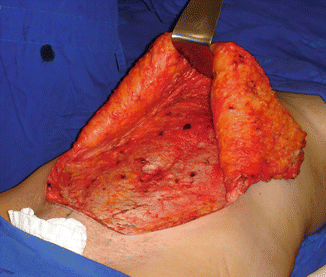
Fig. 43.5
Patient in group II (“control/nonobese”). Skin flap detachment above anterior abdominal aponeurosis
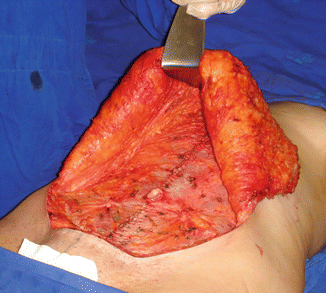
Fig. 43.6
Patient in group II (“control/nonobese”). Approximation (“plication”) of the rectus abdominis muscles
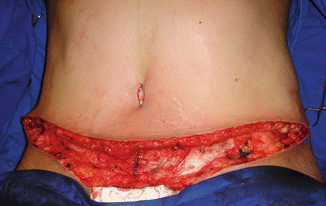
Fig. 43.7
Patient in group II (“control/nonobese”). End of surgery with suprapubic incision
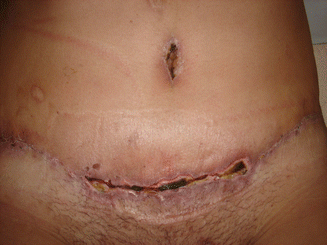
Fig. 43.8
Patient in group II (“control/nonobese”). Skin necrosis in the suprapubic region and dehiscence 22 days after the surgery
Data related to the surgeries of each patient were recorded, as type and duration of each surgery, infiltrated and diuresis volumes, and weight of the removed surgical specimens (WRSS) [1–3]. Samples (biopsies) of subcutaneous cellular tissues from predetermined abdominal regions were also collected (Fig. 43.4), and subsequently an immunohistochemical study with anti-CD34 antibodies was carried out according to the specific methodology described by Souto et al. [3] in 2010.
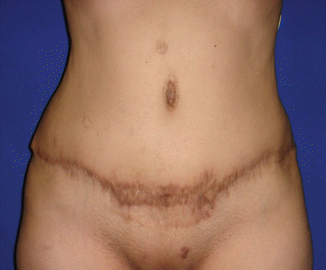
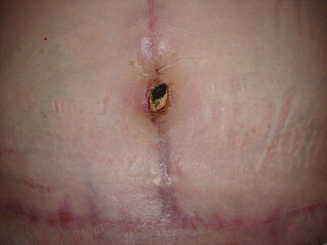

Fig. 43.9
Patient in group II (“control/nonobese”). One hundred and twenty days after healing of skin flap necrosis in the suprapubic region

Fig. 43.10
Patient in group I (“ex-obese”). Umbilical necrosis 45 days after surgery
For the specific evaluation of bleeding volume during surgery, the indirect measure by counting and weighing compresses at the end of the surgery was performed [1–3]. After each surgery, the removed surgical specimen was evaluated based on the weight of the removed surgical specimen divided by the weight of the patient (WRSS/PW) and the weight of the removed surgical specimen divided by the index of body mass of the patient (WRSS/BMI) [1, 3].
43.2.3 Postoperative Period
The volume measure by the drains (Portovac®) was made at intervals of 24 h until the removal of the drains which was performed only when the output of each one was less than 50 mL in 24 h. The complications in the postoperative period were evaluated by observing the clinical conditions and the vital parameters, the surgical incision (scar), and the abdomen aspect of each patient [1, 2].
In order to quantify the vascularization of the sampled adipose tissue of each patient, calculation of three parameters commonly used in the angiogenic evaluation it was carried out: the microvessel density (MVD), the percentage endothelial area (%EndA), and the mean endothelial area (MEndA) [1, 3]. MVD is defined as the number of microvessels per mm2, and %EndA is defined as the percentage of area occupied by endothelial cells. The method used to obtain the results and the statistical tests were described by Souto et al. [3] in 2010.
Regarding the statistical analysis of patients’ data, surgeries, and hematological tests, the results were compared between the two groups (I and II) of patients, and the used methods were described by Souto et al. [2] in 2012.
43.3 Results
Regarding age, there was no statistically significant difference between the patients in groups I and II [1, 2]. Patients in group I presented weight considerably higher and body mass index (BMI) statistically more significant than patients in group II. The average BMI of group I patients was 28.68 kg/m2, ranging from 20.9 to 36.98 kg/m2, and 23.53 kg/m2 for patients in group II, ranging from 21.77 to 26.4 kg/m2 [1–3].
The total duration of surgeries and the total bleeding and the bleeding per hour for each surgery were significantly higher in group I patients [1, 2]. The median of total bleeding was 485 mL for group I and 201 mL for group II [1–3]. In terms of bleeding per hour, the median was 145 mL in group I, with variance of 108 mL, and 65 mL for group II, with variance of 68 mL [1, 2]. There was no significant statistical difference between groups I and II in relation to the volume of daily drainage in the postoperative period, and the average time for maintenance of drains was 14 days [1–3]. The median of daily drainage volume for group I was 134.5 mL with variance of 80.3 mL, and for group II it was 80.3 mL with variance of 89.1 mL [1, 3]. The weight of the removed surgical specimens (WRSS) was higher (statistically significant) for patients in group I [1, 2].
The patients in group II had significantly higher levels of hemoglobin (Hb) only in the intraoperative period; and in the preoperative, immediate preoperative, and immediate postoperative periods, there were no statistically significant differences in Hb levels, and there were no significant differences for any other parameters (hematocrit and platelet count) evaluated in the blood count [1, 2]. There were no significant differences between groups I and II regarding the evaluated coagulometric tests (bleeding time, prothrombin time, activated partial thromboplastin time, and thrombin time) in any of the assessed operative periods.
Patients in group I showed a sharp drop in coagulation factor II values only in the intraoperative period, but there was significant drop in the levels of coagulation factor X in all evaluated operative periods in this group (“ex-obese”) of patients. For all other studied coagulation factors, there were no significant differences between groups I and II in any of the operative periods. Significant differences were not observed between the two groups for any of the thromboelastographic variables studied in the different operative periods [1, 2].
There was no correlation between the studied thromboelastographic variables with thrombin time, prothrombin time, activated partial thromboplastin time, levels of coagulation factors, WRSS divided by the weight of the patient (WRSS/PW), and WRSS divided by the body mass index (WRSS/BMI), nor with the observed total bleeding or the duration of the surgeries. There was no correlation between thrombin time, prothrombin time, activated partial thromboplastin time, levels of coagulation factors, WRSS, WRSS/PW, and WRSS/BMI. However, there was a strong positive correlation of the bleeding (total in ml and in ml/h) observed during surgeries with WRSS, WRSS/PW, and WRSS/BMI [1, 2].
Regarding the immunohistochemical study of the vessels in the subcutaneous tissue and the comparison between the two groups of patients, there were no significant differences related to the microvessel density (MVD), percentage endothelial area (%EndA), and/or mean endothelial area (MEndA). There were no significant statistical differences between the microvessels density values (MVD) obtained from different abdominal areas [1, 3].
Four patients showed postoperative surgical complications, three of them belonging to group I and only one of them from group II. The patient from group II showed necrosis with small area of dehiscence in the suprapubic scar, perceived 14 days after the surgery (Fig. 43.8), and it was considered a minor complication with good resolution in about 45 days after surgery (Fig. 43.9) [1].
Among the patients in group I, there was a case of umbilical scar necrosis perceived 10 days after surgery (Fig. 43.10), requiring prolonged antibiotic therapy and with satisfactory resolution in only 4 months after surgery (Fig. 43.11). Another patient in this group (I) had mild dyspnea and right chest pain in the immediate postoperative period, with radiological diagnosis of atelectasis in the upper right lobe, and she was treated for 48 h with corticoid (hydrocortisone 500 mg IV every 6 h) and respiratory therapy with good evolution and hospital discharge 3 days after surgery [1].
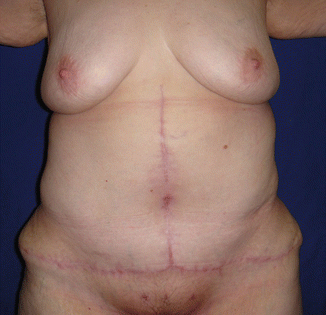

Fig. 43.11
Patient in group I (“ex-obese”). One hundred and twenty days after healing of the necrotic area in the umbilical region
The third case of complication among the patients from group I was considered as a major complication (very serious), and it started at the end of the surgery (Fig. 43.12) when the patient presented hemodynamic instability, and a blood transfusion of a concentrated erythrocyte unity (CEU) was required. There was no improvement in the condition, and the patient remained hemodynamically unstable with coagulation disorder in the immediate postoperative period, requiring transfusion of three CEU. In the first 24 h after surgery, this third patient presented a severe history of coagulopathy, receiving the transfusion of 20 unities of fresh frozen plasma (FFP), eight unities of CEU, and two units of platelet concentration (PLT-C) with high debt by subcutaneous abdominal drain (over 2,500 ml of blood). Then, she was submitted to surgical reexploration, with diffuse bleeding (“drooling”) and lots of clots in the subcutaneous tissue being observed (Fig. 43.13). The patient was referred to the intensive care unit after the new surgery and kept the coagulopathy condition until the tenth day after the first surgery, being submitted to several blood transfusions during this period, eventually progressing to a respiratory infection and death 26 days after the first surgery, by septic shock [1].
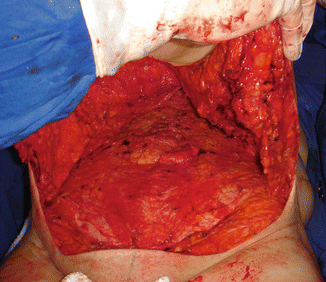
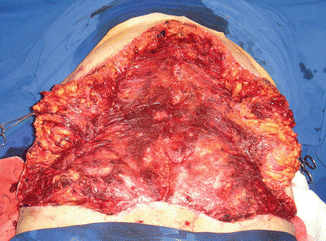

Fig. 43.12
Patient in group I (“ex-obese”). Bleeding (“drooling”) increased and formation of clots during the first surgery

Fig. 43.13
Patient in group I (“ex-obese”). Surgical reexploration 24 h after abdominoplasty (first surgery), showing bleeding (“drooling”) and a large number of clots
43.4 Discussion
Currently, there is almost a consensus that the excessive bleeding that occurs in plastic surgeries carried out in patients that were previously submitted to reducing gastroplasties, with consequent massive weight losses, often implies the need of blood transfusions, and it can cause the formation of hematomas during the postoperative period [19, 20, 26, 34]. However, so far, there are few reliable studies that evaluate and quantify this bleeding [1–3, 17, 26].
The data presented by Souto et al. in 2012 [2] and in 2010 [3] and by Souto in 2009 [1] allow us to state with certainty that increased bleeding occurs during abdominoplasty procedures performed in patients previously submitted to bariatric surgery when compared with patients that were never obese [1–3]. There is no more doubt that there is a major bleeding than expected in “ex-obese” patients (group I) and it is essential to understand why this occurs.
Obesity, even appearing as a residual or recent state, predisposes the organism to systemic inflammatory conditions (proinflammatory state) [35], which may lead to an increase in circulating serum levels of interleukin-6 [36], tumor necrosis factor alpha [37], and C-reactive protein [38]. The increased production of these proinflammatory substances may determine systemic changes such as metabolic syndrome, diabetes, and atherosclerosis through increased resistance to insulin or endothelial dysfunction [39], which could eventually cause changes in hemostasis causing the major bleeding observed in the patients in group I (“ex-obese”). To rule out this possibility and to reduce the risk of interference in the results caused by the recent obesity condition or weight variation in the postoperative period, in 2009, Souto [1] studied only “ex-obese” patients with stable weight for at least 6 months, as recommended by other authors [40, 41].
Prophylaxis of thromboembolic complications with low molecular weight heparin for any of the studied patients was not held [1–3], since there is a 1 % risk of intraoperative major bleeding with the use of this kind of drug [42]. Since smoking is associated with an increased risk of thromboembolic complications [43], all the studied patients were nonsmokers and were operated maintaining the temperature of the operating room at high levels (more than 35 °C) and with intravenous infusion of preheated liquids to reduce the possibility of hypothermia with its harmful effects on blood coagulation [44, 45].
After massive weight loss caused by gastroplasty, histories of intestinal malabsorption are common, with iron deficiency, B1 (thiamine) and B12 (cyanocobalamin) deficiency, protein deficiency, and vitamins A, D, and K deficiency [46–49], which could explain, in the studies of Souto et al. [2] and of Souto [1], the increased bleeding observed in the patients in group I (“ex-obese”). However, all the patients selected for the study of Souto et al. [2] and Souto [1] were submitted to albumin and serum protein dosages and to complete blood counts, in the preoperative period, that did not show any changes. Low serum levels of vitamin K could explain the increased bleeding in patients during surgical procedures [47]; however, the comparison of serum levels of vitamin K performed in the studies of Souto et al. [2] demonstrated higher serum levels in patients in group I (“ex-obese”) when compared with patients in group II (“control/nonobese”), contrary to what could be expected.
The hemoglobin measurements performed by Souto et al. [2] showed lower levels for the patients in group I (“ex-obese”) only in the intraoperative period, and this fact can be explained by the greater loss of blood during the surgeries without contributing for the identification of the cause of the bleeding itself. In the studies of Souto et al., [2] abnormalities or significant differences between the two groups of patients in relation to routine tests of blood coagulation (prothrombin time and activated partial thromboplastin time) were not observed. Often, preoperative tests for blood coagulation, considered as “screening,” may be normal, although there may be a significant reduction of one or more blood coagulation factors [22]. In this regard, it was important to carry out the specific dosage of each one of the blood coagulation factors in the two groups (“obese” and “controls”) to reveal any changes that could exist and were not detected by the “screening/routine” tests.
Regarding the specific factors of blood coagulation, Souto et al. [2] and Souto [1] found lower levels of factor II (prothrombin) in patients of group I (“ex-obese”) only in the intraoperative period and significantly lower blood levels of factor X in patients of group I in all the studied operative periods. Factors II and X of coagulation are both vitamin K dependent [50]. This could again lead to the hypothesis of major bleeding observed in surgeries of patients in group I being caused by vitamin K deficiency. However, dosages of factors VII and IX of coagulation, also dependent on vitamin K, showed no deficiencies or significant differences between the two groups of patients studied by Souto et al. [2]. Additionally, the specific dosages of vitamin K made by Souto et al. [2] showed blood levels of vitamin K significantly higher in “ex-obese” patients, definitely ruling out the hypothesis of increased bleeding observed in these patients being due to vitamin K deficiency.
As factor II (prothrombin) is consumed during states of increased activation of coagulation and factor X is activated in the common coagulation pathway, triggering thrombin generation and subsequent fibrin formation, and it is also more consumed during hypercoagulable states, one strong possibility is that the patients from group I (ex-obese) studied by Souto et al. [2] and by Souto [1] presented, during surgeries, increased activation of the coagulation “cascade” [51], leading to the depletion of blood coagulation factors [52]. However, to confirm this theory, changes in the thromboelastography or in bleeding correlations observed with greater tissue trauma caused during surgeries of patients in group I would be expected.
Thrombin is generated from interactions of several coagulation factors, cells, and platelets present in the blood, and the polymerization of fibrin is the physiological consequence of thrombin generation [53]. Thromboelastography, expressing the coagulation process through the analysis of whole blood, indirectly reflects the course of thrombin generation and provides detailed information on hemostasis in real time [54].
In the studies by Souto et al. [2] and Souto [1], there were no statistically significant or consistent differences between groups I and II in the evaluated parameters in thromboelastography during the evaluated operative periods. However, as in general, there is a poor correlation between routine tests (prothrombin time and activated partial thromboplastin time) of coagulation and thromboelastography [55]; it was important to conduct both exams in the studies of Souto et al. [2] because thromboelastography provided additional information on hemostasis, especially regarding fibrinolysis [56], allowing to rule out the hypothesis of blood clotting disorders that could cause the major bleeding observed in “ex-obese” patients [1, 2].
It is then necessary to consider the hypothesis that there may be greater subcutaneous vascular bed/mesh in “ex-obese” patients as proposed by Baptista et al. [27] in 2009. Angiogenesis favors perfusion of the involved tissue [57], improves the supply of oxygen and nutrients, and facilitates dissipation of final products of cellular metabolism [58]. The higher the proliferation of a particular tissue, the greater are its metabolic needs, and therefore, the degree of angiogenesis should be more significant [59]. Despite being metabolically little active, the adipose tissue in the obese subject is the result of a hypertrophy/hyperplasia process whose maintenance requires a concomitant expansion, even disproportionally smaller, of the nurturing vascular bed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







