(1)
Department of Dermatology, Drexel University, Philadelphia, Pennsylvania, USA
Abstract
The immunology of eczema involves an interaction of adaptive and innate immune responses. Toll-like receptor 2 (TLR2) activity drives the reaction that ultimately results in an eczematous lesion. Pathways leading towards inflammatory responses include the MyD88 pathway, which can be set in motion by nearly all the cells in the epidermis. This pathway leads to nuclear factor-κB, which mediates inflammation in part by the production of tumor necrosis factor α (TNFα). TNFα is considered the most important mediator of spongiosis, which is the leading pathological finding. Also important is the stimulation by TLR2 of PAR2 (protease-activating receptor 2), the molecule that is the leading pruritogen in eczema.
Keywords
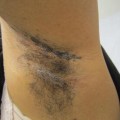
Adaptive immune systemIgEInnate immune systemMild atopic dermatitisMildly impetiginized atopic dermatitisMyD88Nuclear factor-κBPAR2 (protease-activating receptor 2)Severe atopic dermatitis Staphylococcus aureus Staphylococcus epidermidis Toll-like receptor 2 (TLR2)Tumor necrosis factor αClinical photographs associated with the immune response include mild atopic dermatitis, which results mostly from the innate response (Fig. 1.6); mildly impetiginized atopic dermatitis, which results from a combination of innate and adaptive responses (Fig. 4.1); and severe atopic dermatitis, in which the innate response is overshadowed by the adaptive response (Fig. 4.2).
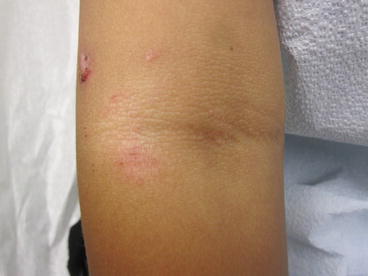
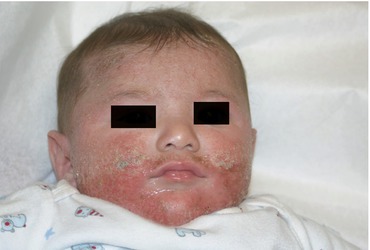

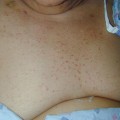
Fig. 4.1
Pink-crusted plaques are present in the antecubital space; a small pustule is noted, as are focal hemorrhagic crusts. This represents both an innate and an adaptive immune response

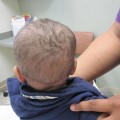
Fig. 4.2
A severe, weeping, oozing honey-crusted eruption is present on this child’s face. This represents an innate, but mostly adaptive immune response (because of the impetiginization noted)
Atopic dermatitis is part of a triad including seasonal allergies and asthma and, by many, is considered the initial step in the allergic “march” that ends with asthma [1]. Where all these disorders are unerringly linked with IgE (the allergy immunoglobulin), eczema has been considered to be driven by reactive IgE. When Staphylococcus aureus was discovered on lesional skin, this concept was further refined to include IgE linked to that organism [2]. Both IgE and S. aureus IgE are part of the “adaptive” immune system, which is either B lymphocyte derived with immunoglobulins or T lymphocyte derived with cell-mediated immune functions [3].
T-cell-mediated immunity can involve cytotoxic reactions that occur when cytotoxic CD8 cells inflict direct damage to particles. T4 helper cells can secrete cytokines that activate CD8 T cells and recruit and activate both monocytes and macrophages, which then damage microbiologic and other particles. These cytokines include interleukin-2, interferon, tumor necrosis factor (TNF) α and β, monocyte chemotactic factor, and others.
This also is not to deny that adaptive immunity plays a very large role in atopic dermatitis [4]. Soon after the very first scratch that leads to the plaque of eczema (remember this disease is the “itch that rashes”), the adaptive immune system activates and aids considerably in the perpetuation of lesions. It is what occurs “before” that first itch/scratch that has always intrigued us.
The Adaptive Immune System
The classical pathway of the adaptive immune system, with B-cell humoral immunity, involves antigen-antibody binding that activates complement and leads to cytolysis of particles, agglutination, opsonization, and chemotaxis. The reaction can then be cleared by macrophages and neutrophils.
On pathology, as we noted in the previous chapter, eccrine sweat ducts become occluded with periodic acid–Schiff (PAS)-positive material. Gram-positive organisms are present in ducts as well. Our original hypothesis for the PAS-positive material was that it was biofilm produced by the gram-positive Staphylococcus epidermidis [5]. We had observed this biofilm directly by light and confocal microscopy in specimens taken from lesional skin [5]. Control skin (away from lesions), or healed skin where eczematous plaques previously resided, had no biofilms visible on either type of microscopy. With the recognition that many other staphylococci that made biofilms were involved, our hypothesis was expanded to include all biofilm-producing staphylococci.
We postulated that the innate immune system was reacting to the presence of this biofilm produced by these staphylococci. Further, where they are gram-positive organisms, we also considered that the likely reactant of the innate immune system would be Toll-like receptor 2 (TLR2), which reacts with gram-positive organisms in its role as a defender in the innate immune system [4] (Fig. 4.3).
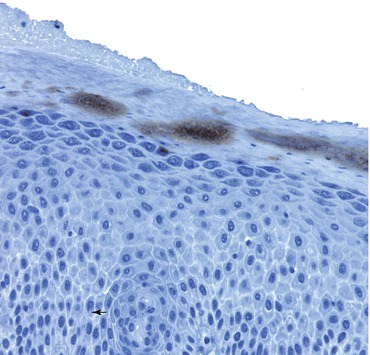

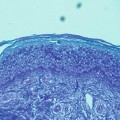
Fig. 4.3
In this section of skin from lesional atopic dermatitis, Toll-like receptor 2 (TLR2s) are noted periductally in the stratum corneum. The ordinary location for TLR2 is in the basal zone of the epidermis. Activation of TLR2 is noted in the proximal midstratum corneum, adjacent to the occlusions noted on hematoxylin and eosin, periodic acid–Schiff, and Congo red staining. TLR2s react to gram-positive bacteria, such as staphylococci or streptococci
Innate Immunity as Seen and Postulated in Our Findings
Gram-positive bacteria and biofilm → TLR2 → MyD88 → NF–κB → TNFα → spongiosis
Gram-positive bacteria and biofilm → TLR2 → kallikrein → PAR2 → pruritus
We stained immunohistochemically specimens that had shown PAS-positive ductal occlusions with TLR2 antibody to observe any reactions. We noted staining periductally in the proximal stratum corneum in the lesional skin (where the occlusions were located). Control (nonlesional) skin showed staining in the basal zone of the epidermis. Consequently, TLR2 receptors were being activated right at the site of the ductal occlusion in eczema. Coincidentally, in a recent work measuring Toll receptor response to treatment, some investigators had unrecognized ductal staining of TLR2 in their specimens of lesional skin [6]. After treatment, the only staining was in the basal zone (as in our control). This helps confirm the validity of both our lesional and control skin observations inasmuch as their specimens showed the same findings as did ours.
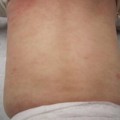
In severely impetiginized lesions (Fig. 4.2), the TLR2 staining was more diffuse and was concentrated in the stratum granulosum (Fig. 4.4). Of interest, this is the same area of the epidermis in which the cleft occurs in bullous impetigo and pemphigus foliaceous, which is associated with desmoglein 1.
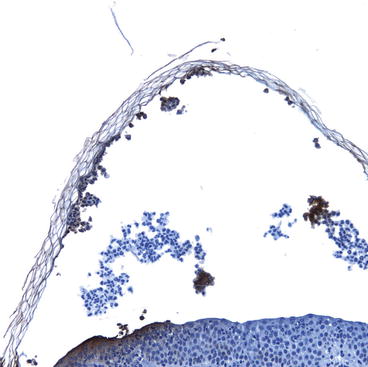

Fig. 4.4
In this section of skin from severely impetiginized atopic dermatitis, activation of Toll-like receptor 2 (TLR2s) is noted in the upper epidermis and the partially separated stratum corneum. This area is also the area involved in bullous impetigo
Once the TLR2 activation is noted, the pathways leading towards inflammatory responses are well mapped [4, 7]. Prime among them is the MyD88 pathway, which can be set in motion by nearly all the cells in the epidermis [8]. The end result of this pathway carried out in these cells is nuclear factor-κB (NF-κB), an incredible molecule that mediates inflammation in part by the production of TNFα [9]. (NF-κB also mediates many other inflammatory and noninflammatory processes.) TNFα is considered the most important mediator of spongiosis; this brings us full circle to the well-established finding that eczema pathologically is caused by spongiosis [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree