Radiolucent lesions
High-density lesions
Lipoma
Phyllodes tumour
Cyst lipoid
Cyst
Galactocele
Abscess
Mixed density lesions
Hematoma
Fibroadenolipoma
Lymphadenopathy
Galactocele
Atheroma
Intramammary lymph node
A differential diagnosis may be required with
Hematoma
Carcinoma (medullary, solid)
Low-density lesions
Atheroma
Fibroadenoma
Sarcoma
Cyst
Metastasis in breast
Giant fibroadenoma
Phyllodes tumour
Mucinous carcinoma
Papillary carcinoma
Abscess
Cavernous haemangioma
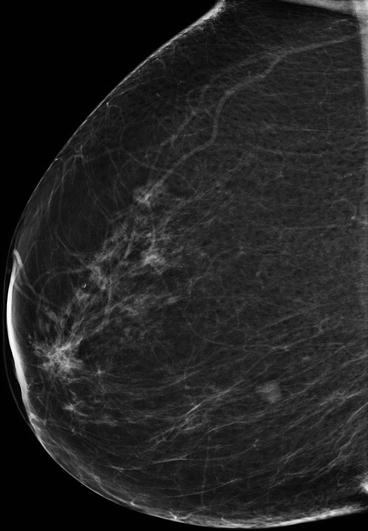
Fig. 5.1
Probably benign mass with mostly circumscribed margins. A core-needle biopsy (CNB) should be performed to exclude malignancy. In the case of negative result, follow-up should be planned to establish the long-term stability
FINDINGS SUGGESTING MALIGNANT DISORDERS. Findings suggesting malignant disorders are listed in Table 5.2. Ill-defined borders, spicules and distortion are the main characteristics of a malignant mass (Fig. 5.2). Well-defined but lobulated borders are an intermediate feature, shared between benign and malignant lesions.
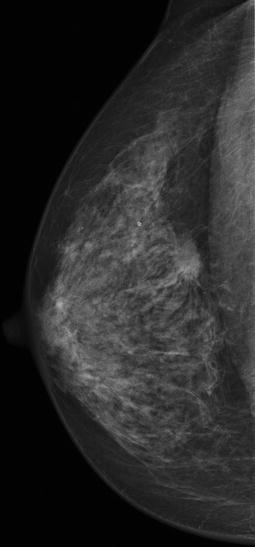
Table 5.2
Findings suggesting malignant disorders
Main findings |
Solid mass with ill-defined borders |
Irregular or spiculated mass |
Architectural distortion |
Less common findings |
Enlarging solid, well-circumscribed mass |
Developing density compared with previous films |
Focal asymmetric density |
Skin thickening |
Nipple retraction |
Enlarged and dense axillary lymph nodes |
Spiculated abnormality should be considered with: |
Postsurgical or radial scar (benign) |
Area of fat necrosis |
Apparently normal asymmetric breast tissue with or without evidence of architectural distortion (constitutional in 3 % of healthy women) |
Some typical semiological findings |
Infiltrating ductal carcinoma: high attenuation mass with spiculated margins and focal distortion |
Infiltrating lobular carcinoma: focal distortion with inhomogeneous density |
Ductal carcinoma in situ: cluster of pleomorphic microcalcifications |
Pure mucinous carcinoma: circumscribed opacity with distinct margins |
Medullary carcinoma: lobulated opacity circumscribed oval or round |
Inflammatory carcinoma: diffuse thickening of trabecular density diffusely increased |
Metastases from extramammary primitiveness: round opacity with distinct margins |

Fig. 5.2
Mammogram of a typical BC: irregular, dense, spiculated mass lesion, more conspicuous on post-compression view and associated with architectural distortion. No suspicious microcalcifications are noted
Spicules represent fibrous reaction to the growth of the mass that usually is dense in the centre. Histologically, the tumour may or may not extend along the spicules. Spiculated abnormality should be considered also to be associated to postsurgical or radial scar (benign) and area of fat necrosis. Apparently normal asymmetric breast tissue could be suspicious also without evidence of architectural distortion, even though it can be found in 3 % of healthy women.
Small malignant findings (Fig. 5.3), if surrounded by connective tissue and without calcifications, are mammographically detectable only when they are located at the edge of the breast parenchyma or cause parenchymal asymmetry and/or skin/nipple retraction. Also calcifying diffuse malignancies could be missed by the radiologist, unless additional mammograms or ultrasound are performed.
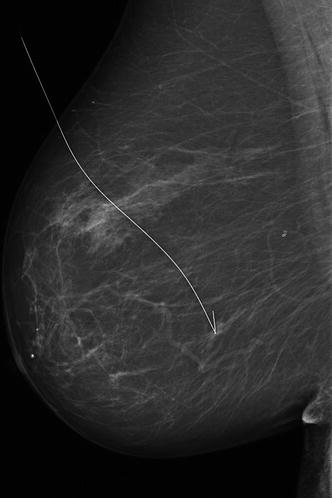

Fig. 5.3
A typical clinically occult BC detected on a screening mammogram. A small mass with ill-defined irregular margins. The spot compression magnification provides improved resolution and shows a margin which is clearly spiculated with microcalcifications that may not have been visible on the standard image. Mandatory surgical biopsy requires a hook-wire preoperative localisation, as shown in the mammogram
CALCIFICATION. Calcifications are another characteristic of cancer. Calcifying neoplasms, unlike non-calcifying cancers, are radiographically detectable at a relatively early stage, long before they are manifested clinically and also long before they exhibit malignant features at ultrasound or MRI. These relatively slow-growing lesions are definitely in the domain of mammography, and because of this, mammography is still essential for the early detection of BC. The most important elements in the analysis of calcifications are morphology and distribution, but also appearance of calcifications over time.
Morphology. Rounds, rings, needles and parallel lines are morphological features of benign lesions, due mainly to sediments of calcium to the bottom of cysts, to the calcified fibrous tissue or to vascular calcifications that have distinctive parallel track appearance. Large calcifications (popcorn-like) in a mass more likely correspond to a benign lesion such as an involuting fibroadenoma or papilloma.
Calcifications associated with malignancy are <0.5 mm in diameter and vary in size and shape. Most typical calcification associated to intraductal carcinoma are pleomorphic, linear and branch-shaped (as expression of casting duct) and/or granular (Fig. 5.4).
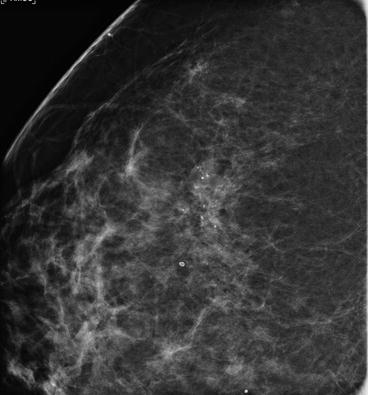

Fig. 5.4
Area of pleomorphic microcalcifications (magnified view), partly distributed within linear branching arrays. A classic high-grade intraductal carcinoma with calcified necrotic tumour debris within the duct
Distribution of benign calcification is in large round areas, while malignant ones have a clustered, linear or segmental distribution. A synopsis of diagnostic features of calcification is shown in Table 5.3. Note that the presence of typical benign calcification does not exclude coexistent malignancy and each area of calcification within a breast should be judged separately.
Table 5.3
Diagnostic features of radiological calcifications
Feature | Benign | Malignant |
|---|---|---|
Distribution | Single, areas or diffuse | Clustered (>5 in a small area) |
Cluster shapes | Round | Linear, triangular, rhomboid |
Form | Rounds, popcorn, rings, needles, tea cups, parallel lines | Casting (linear and branching), granular, pleomorphic |
Size | Uniform | Variable |
Density | Uniform | Variable within and between calcifications |
Appearance over time. Intraductal neoplasia (or intraductal component of an infiltrating BC) should be suspected whenever a new microcalcification cluster appears within a short period of time. If the nature of clustered microcalcifications remains uncertain, it is better to recommend a core-needle or vacuum biopsy under digital stereotactic guidance than the usual short-term follow-up.
The biopsy can be done quickly on an outpatient basis, is well tolerated and can provide a definitive diagnosis. It also relieves patient anxiety by eliminating the waiting time for future follow-ups. Moreover, follow-ups are often unrewarding because benign adenosis and particularly low-grade in situ carcinomas calcify very slowly over a period of years or even decades and the calcifications may regress or disappear completely on progression to an invasive neoplasm. Short-term follow-up tends to increase more than allay the patient’s (and doctor’s) level of uncertainty.
5.1.4 Mammography in Special Situations
Calcifications. Unilateral magnification mammogram (magnification compression views) is the primary technique for further investigation of calcifications. Geometrically magnified images can provide better image quality and, therefore, more accurate diagnosis than electronically zoomed images.
Tissue sampling. It is always advisable to obtain the mammogram prior to any microinvasive procedure. After core-needle biopsy, it is recommended to wait 2 weeks or more so that tissue changes (as hematoma) can resolve.
Surgery. Being sure to acquire mammograms prior to surgery is essential. After surgery mammography to determine need for re-excision should wait 2–3 weeks for oedema to resolve. Focal skin thickening and retraction at surgical site together with variable amount of distortion and/or mass at the tumour bed jeopardises the results.
Radiation therapy. After radiation therapy, oedema is usually distributed in the gravity-affected portions of the breast, mostly periareolar and inferiorly. Coarse trabecular changes and early calcifications of fat necrosis may be indistinguishable from malignancy. Infrequent oil cysts are observed.
Follow-up. To evaluate for new mass/calcifications, a new baseline mammogram 6 months after radiation therapy should be obtained. Lumpectomy site should always be followed up with magnification, and presence of calcifications or unusual distortions take close-up views every 6 months for the first 3 years after surgery. However, except in justified cases, short-interval mammography after conservative breast surgery adds little benefit and dramatically increases material and psychological costs.
5.2 Ultrasound Scanning
Clinical Practice Points
Ultrasound scanning (US) is a diagnostic rather than a screening procedure, more targeted for the study of localised breast lesion than for an overall view.
Since US is less affected by breast density, it shows a better diagnostic performance than mammography in symptomatic young patients and in women with radiographically dense breasts.
US is the most operator-dependent of all imaging modalities.
US guided localisation and sampling is a first-choice diagnostic tool in most cases.
US may not replace mammography in women older than 40 years, but may equally give an important contribution to diagnosis.
5.2.1 Overview
Besides mammography, ultrasound is the technique of choice for further investigation of focal symptomatic breast problems at all ages. Under 35 years of age, when the risk of BC is very low, it is usually the only staging technique required. Over 35 years of age it should be used in association with mammography.
Ultrasound is less sensitive than mammography for the early signs of BC and is therefore not used for screening. However, ultrasound does increase detection of small BC in women who have a dense background pattern on mammography. Moreover, ultrasound is the technique of first choice in the guidance of biopsy of both palpable and non-palpable breast if visible on scanning. The characteristic and main indications of breast ultrasound are shown in Table 5.4.
Table 5.4
Ultrasound scanning advantages and diagnostic features
The best method to differentiate solid vs. cystic lesions which have: |
Circumscribed margins |
Sharp anterior and posterior walls |
No internal echoes |
Posterior enhancement |
The best method to guide tissue sampling by FNA and CNB |
Easy performing aid: |
In benign-malignant differentiation of solid nodules |
In determining BC: |
Skin invasion |
Superficial fascia involvement |
Muscle invasion |
Findings suspicious for malignancy |
Solid hypoechoic non-oval area with taller (antiparallel or perpendicular to the skin) than wider (parallel to the skin) span that may denote fast growth and invasion of tissue |
Irregular margins |
Acoustic shadowing, associated with malignancy in about 60 % of cases |
Anterior echogenic rim, sometimes corresponding to desmoplastic reaction caused by invasion |
Multiple lobulations (>4 lobules) |
Abnormal vascularity found in Doppler ultrasound |
Inside calcifications, although poorly characterised |
Ultrasound is an easy performing aid in differential diagnosis between cystic and solid nodules, as well as in differentiation between benign and malignant nodules.
CYST. Sonographic features of a simple cyst include well-circumscribed shape, smooth walls with sharp anterior and posterior borders and anechoic signal with posterior acoustic enhancement. Cysts may display calcifications in their periphery and do not increase in size in postmenopausal women, unless they use HRT.
All cysts that do not meet strict criteria are, by default, classified as complex cysts. The vast majority of complex cysts fall within the broad spectrum of fibrocystic change. For these reasons, the majority of complex breast cysts are not worrisome and do not need to be aspirated or biopsied [3]. With the improved resolution of current high-resolution equipment, a large percentage of breast cysts appear complex or dirty. This is because there is real stuff within most breast cysts, due to fibrocystic change. With older equipment these internal cells and debris were not visible, and the cysts appeared simple. Internal contents within breast cysts are part of the spectrum of fibrocystic change and include protein globs, cellular debris, cholesterol crystals, foam cells and apocrine cells, floating and papillary.
FINDINGS SUGGESTING BENIGN DISORDERS. Benign characteristics of solid nodules are as follows:
Well-circumscribed, slightly hyperechoic homogeneous structure
Wider than deep morphology with major axis parallel to the plane of the skin
Regular gently curving smooth polilobular (<3) margins
Thin echogenic pseudocapsule in a wider than deep nodule that probably represents normal compressed tissue consistent with a non-infiltrative process
Lesion contour and shape are considered to be the main features that allow in differentiating benign and malignant lesions, the former with high sensitivity and the latter with high specificity. When one aspect does not agree with other findings, tissue sampling (FNA or CNB) is mandatory (Fig. 5.5).
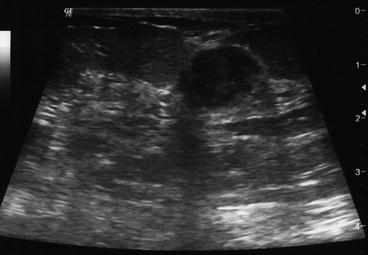

Fig. 5.5
Ultrasound scan of one lesion with prevailing, but not all, features of benignity: relatively uniform hypoechoic mass, quite delimited margins, transverse diameter wider than taller, posterior acoustic enhancement and no calcifications. According to age, a core-needle biopsy (CNB) should be performed to exclude malignancy
Among solid nodules, the appearance of intraparenchymal lymph nodes, small, round or kidney-shaped, homogeneous to the periphery with echogenic hilum is typical.
FINDINGS SUGGESTING MALIGNANT DISORDERS




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








