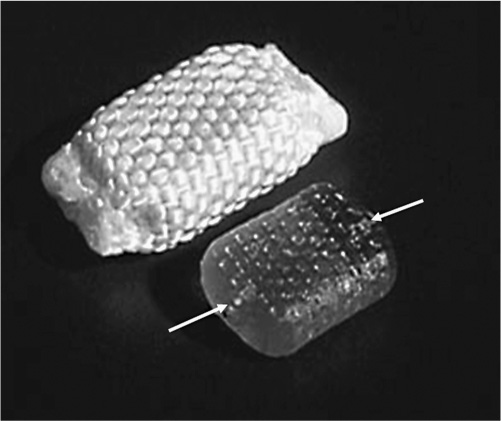
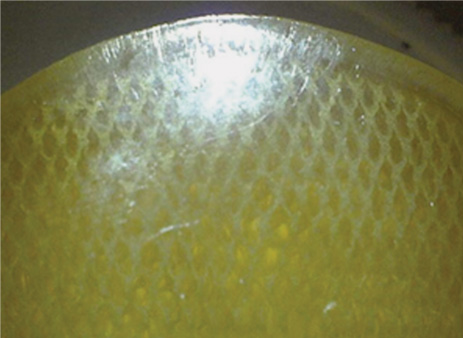
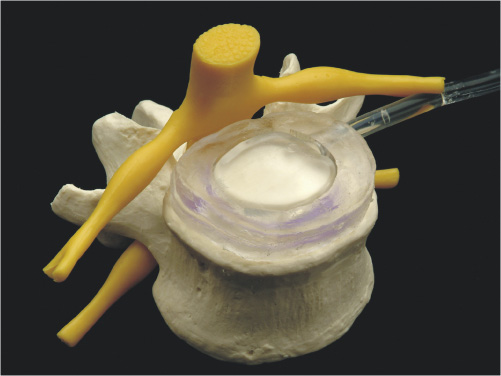
1 Advancements in nonfusion technology for the treatment of degenerative and damaged spinal disks and associated conditions have ushered in a new era of spine care. Commonly referred to as spinal arthroplasty and dynamic stabilization, this new technology is unlike traditional fusion methods (spinal diskectomy and arthrodesis), which purposely impair normal motion by disrupting articular surfaces and locking two or more spinal vertebrae to function as a single unit. Nonfusion techniques aim to provide stabilization while maintaining the mobility and function of the spine and eliminating the pain caused by the damaged spinal disks. Current research efforts and increasing interest in new motion and spine preservation technologies are certainly indications that the traditional spinal fusion approach will perhaps one day no longer be the gold standard for treatment. The traditional fusion methods for treating symptomatic segmental disk disease, which include diskectomies (decompression) and arthrodesis (with or without decompression), are considered the standard of care in many instances. However, there are numerous problems and disadvantages generated from such procedures. Among these issues are loss of spinal mobility and flexibility, permanently altered motion characteristics and biomechanics, grafting collapse resulting in suboptimal sagittal balance, and autograft harvest site pain. In addition, one of the most prevalent concerns following fusion at one or more levels is the transference of stress to the adjacent levels, which often results in repeat surgeries, other complications, and more pain.1–3 The new motion preservation technologies of spinal arthroplasty and dynamic stabilization offer significant advantages, including the maintenance of range of motion and mechanical characteristics, restoration of natural disk height and spinal alignment, significant pain reduction, and prevention of adjacent segment degeneration. The concept of motion and preservation of the spine has been around for more than 50 years. However, the most significant advances have been made within the last 2 decades. Artificial disks have been in clinical use in Europe since the late 1980s and rapidly became a feasible treatment option during the 1990s. Since 2000, arthroplasty for both lumbar and cervical degenerative disk disease has been introduced in the United States for clinical trials. Efforts in dynamic stabilization technologies are quickly advancing, and in 2004 the U.S. Food and Drug Administration (FDA) approved the first lumbar arthroplasty device (SB Charité III, DePuy Spine, Raynham, MA) for use in the United States4 (Fig. 1–1). This chapter reviews the historical development of spinal arthroplasty and dynamic stabilization to provide some understanding of its evolution. Due to the overwhelming number of reports and studies conducted over the decades, this chapter does not attempt to list all of them but rather to highlight a handful and summarize the rest. Figure 1–1 Charité Artificial Disk. Nonfusion spinal technology encompasses spinal arthroplasty devices (which replace part or the entire disk with an implant that imitates the functions of a normal disk and allows natural motion), and dynamic stabilization devices (which preserve the intervertebral disk and vertebral structure by attaching a device to the back of the spine to help stabilize the motion segment while leaving the intervertebral disks intact). Numerous attempts have been made over the last several decades to design effective spinal disk replacement devices. The progress of development in artificial disk technology may seem slow in comparison with other artificial joint technologies for the knee and hip. However, the distinction is not rooted in a lack of need, initiative, or effort, but rather in the complexities involved with the research and development process such as the design, surgical approaches and techniques, and patient selection, as well as the complexity of the structure and function of the spine itself.2,5 The intervertebral disk is considerably more complex because it is composed of three distinctively different tissues—annulus, nucleus, and end plate—and performs a dual function in maintaining spinal column stability while also providing the column with necessary flexibility. Therefore, much more consideration is required in designing an effective and reliable artificial disk that will maintain proper intervertebral spacing, allow for motion, provide stability, and bear loads. In general, there are two types of spinal arthroplasty devices: nucleus replacement (replacing the nucleus only) and total disk replacement (replacing the entire disk). In a nucleus replacement, only the inner portion of the disk (the nucleus) is removed and replaced with an implant. Ongoing research into various designs for replacements has incorporated the use of metal, ceramic, hydrogel, elastic coils, and other similar materials. Another promising replacement design consists of injectable polymers that are cured in situ to form a nucleus prosthesis, which has the desirable element of delivery via a minimally invasive, outpatient procedure. In comparison with total disk replacement, the primary advantage to this option is that it preserves the annulus and end plates while replacing only the defective portion of the nucleus.6 In a total disk replacement procedure, all or most of the disk tissue is removed and an entirely new device is implanted into the space between the vertebra. The many designs being studied for artificial disks incorporate metal, polyethylene, polyurethane, and other biomaterials or combinations of materials. The most commonly used design incorporates two plates that are anchored to the top and bottom surfaces of the vertebrae, above and below the disk being replaced, along with some type of compressible plasticlike piece between the plates. A total disk replacement would be used when all elements must be removed, including the nucleus and annulus, to remove the pain and treat the defective area.7 In the late 1950s, initial attempts at replacing the nucleus pulposus space involved implanting polymethyl methacrylate (PMMA), silicone, or stainless steel ball bearings.8–10 Fernström attempted to preserve motion by inserting stainless steel ball bearings into the intervertebral disk space areas of the cervical and lumbar spine in an effort to reproduce the “ball joint” mechanism of the disk.10 Unfortunately, the results were not very promising. McKenzie also published preliminary and long-term clinical study reports of this device reporting reasonably good results; however, its use has been discontinued because of concerns regarding subsidence and migration.2,11,12 During this same period, Nachemson13 performed biomechanical testing to demonstrate the relative restoration of some disk properties by injecting selfhardening silicone rubber into cadaver disks. He also tried silicon testicular prostheses but found that the implants rapidly dissolved after 20 to 30 thousand cycles of walking load.9,13,14 Similarly, Hamby and Glaser tried injecting PMMA into the disk, which resulted in flow control problems.8 In 1973, Urbaniak et al used an injectable mixed silicon Dacron device in nonhuman primates and reported bone resorption along with aberrant bone formation.15 In 1974, Schneider and Oyen performed experimental work on silicon nucleus replacement that was similar to Nachemson’s work in the 1960s.17 In an effort to replicate the natural properties of the nucleus, in 1975 Froning developed a discoid device with a central, collapsible bladder to be fixated to the vertebrae with a spike.18 In 1977, Roy-Camille et al tried to contain medical grade silicone in a latex bag while injecting into a human cadaveric disk.19 Fassio and Ginestie designed and patented an elastic disk that had a Silastic central sphere and was bordered by a lateral plateau in an uncompressible synthetic resin.20 They subsequently reported the first clinical study of the silicon nucleus using a monkey model, followed by human implantation into three patients in 1977. Follow-up reports showed a marked disk narrowing and absence of motion in all patients because the device had subsided and migrated into the vertebral body of all patients.21 Later, Horst improved the design of the device, which had better positive locking and more uniform stress distribution.22 In the early 1980s, research efforts continued. Hou conducted biomechanical and animal studies in a monkey model and subsequently implanted a silicone prosthesis in more than 30 patients.23 The results from this study were not published. In 1981, Edeland suggested that the principle of hydrophilic-hydroelastic intervertebral interposium be used as a nucleus replacement after diskectomy surgery. He described a disk containing a hydroscopic agent, which would be used to expand the disk after introduction.24 In 1982, a nucleus replacement device was developed and patented by Kunze.25 He designed a fish-shaped device with a fin-type tail with transverse grooves to provide a friction fit in concert with the broadened tail. Research into reproducing both the mechanical and the physiological properties of the nucleus was accomplished by Bao and Highman, who subsequently developed a hydrogel intervertebral nucleus that contained ~70% water content under physiological loading conditions. Similar to natural nucleus material, the hydrogel contained the required mechanical properties and the ability to absorb and release water with changes in the applied load. Biomechanical studies have confirmed the restoration of disk anatomy after the implantation of this device.26,27 Figure 1–2 Photograph of the PDN-SOLO implant showing the internal copolymeric pellet (lower figure) and the intact implant with its woven jacket of high molecular weight polyethylene (HMWPE). The arrows at the ends of the pellet indicate the internal location of the x-ray visible Pt-Ir wire stubs. In 1988, Ray developed a nucleus replacement composed of dual-threaded cylinders with an ingrowth fiber capsule, an injectable thixotropic gel that would swell from a collapsed state.28 However, technical problems in manufacturing and development led to concept changes. Subsequently, in 1990, Ray developed the Prosthetic Nucleus Device (PDN) (Raymedica, Inc., Bloomington, MN), which consisted of a hydrogel core enclosed in a woven polyethylene jacket resembling a pillow29,30 (Fig. 1–2). The hydrogel contained hydrophilic properties to imitate the behavior of the nucleus pulposus. After several device migrations, additional design modifications were made.31 The PDN device marked a new era in the development of nucleus replacement devices. Since 1996, the device has experienced widespread use in humans, with more than 4000 patients implanted internationally to date (with the PDN or the most recent PDN-SOLO device).32 Clinical results from the beginning have been encouraging.33 The first 10 PDN devices implanted in Germany in 1996 reported only one extrusion. By 2002, there were 480 procedures recorded with a removal rate of 5%.34 The most recently updated version, the single PDN-SOLO device, shows considerable improvement over the initial paired PDN devices, and its methods of implantation have had good to excellent results.32 Newcleus (Centerpulse Spine-Tech, Minneapolis, MN), another nucleus replacement device implanted into humans, is a stand-alone device made of polycarbonate urethane curled into a preformed spiral and inserted via an open technique. This device was implanted into five patients in a pilot study.35 The follow-up reports indicated no failures, migration, or complications, and retained disk motion was documented on plain x-rays with facet function monitored using a computed tomography scan.36,37 The Aquarelle (Stryker Spine, Allendale, NJ) is another hydrogel-based nuclear replacement that is composed of a polyvinyl alcohol, which is hydrated to a physiological water content of ~80% prior to implantation.1,15 Once it reaches physiological equilibrium, the implant expands and contracts freely in situ. Testing indicated that extrusion occurred during in vitro testing only under loads well in excess of those expected in vivo.38 The NeuDisc SNI Hydrogel Polymer (Replication Medical, Inc., New Brunswick, NJ) has recently been reported as a hydrogel that imbibes water and expands preferentially in the axial direction39 (Fig. 1–3). The Prosthetic Intervertebral Nucleus (PIN) (Raymedica, Inc., Bloomington, MN) is an in situ curable polyurethane that is injected into a balloon catheter delivery system.40 The curable protein hydrogel cures within minutes in the disk space, after which the catheter is removed. The BioDisc (Cryolife, Kennesaw, GA) is an injectable protein hydrogel device based on Cryolife’s surgical adhesive product. Early bench work fatigue testing at 10 million cycles indicated a 10% loss of disk height, which later recovers.37 The Injectable Disc Nucleus (IDN) (Spine Wave, Shelton, CT), is a hydrogel composed of synthetic silk-elastic copolymer produced through DNA bacterial synthesis fermentation, which cures within a few minutes. Early test results indicate that disk height is restored under load, and the material resisted extrusion during cadaveric mechanical testing.37 Figure 1–3 Flexion and extension motions about the major axis of the NeuDisc were performed to a simulated life of 10 years. Three of the flexion-extension samples were removed from endurance testing to be evaluated against controls. The remaining three samples proceeded to lateral bending testing. The three flexion-extension endurance samples removed for evaluation showed no signs of delaminations, tears, cracks, or major surface defects. All three samples had small wear lines oriented radially from the major axis. Figure 1–4 DASCOR Disc Arthroplasty Nucleus Replacement Device shown in a spine model. The in situ curable polymer is injected into a polyurethane balloon placed in the disk space. The balloon expands to fill any void that has been created during the diskectomy. The polymer cures in a matter of minutes from a liquid to a firm but pliable state. The DASCOR (Disc Dynamics, Eden Prairie, MN) is an injectable cool polyurethane polymer that cures in situ within 12 to 15 minutes using a balloon, which is utilized to create space in the disk for the delivered material6 (Fig. 1–4). There are numerous other ongoing development studies under way, with new research efforts that will undoubtedly continue on in the future. Among these are a one-piece ceramic or metal implant that anchors to the inferior vertebral body as a hemiarthroplasty (being developed by Interpore Cross International, Irvine, CA); a hydrogel memory coiling material that imbibes fluid to restore disk height (by Mathys Medical in Switzerland); and a chemonucleolysis product to identify a formulation and dose to initiate the regeneration process of the nucleus pulposus (by NuVasive, Inc., San Diego, CA). Total disk replacements can generally be categorized into two types: lumbar and cervical. Although initial efforts by Fernström in the late 1950s were not promising, much has been learned since then. During the 1980s, a renewed resurgence of interest in spinal arthroplasty brought about a flood of studies and research efforts. Steffee, who had been involved in designing artificial disks since the mid-1970s, made substantial advancements with the development of high-density polyethylene (PE)/CoCr prosthesis. This led to the development of the AcroFlex (DePuy Spine, Raynham, MA) device in the mid-1980s, which was composed of a polyolefin-based rubber core sandwiched between titanium end plates and was subsequently used in six patients between 1988 and 1989.41 However, because of disintegration of the rubber core in the preliminary clinical trials, further efforts were suspended. In the 1990s, Steffee developed the second-generation version of the AcroFlex (DePuy Spine) that was similar to the first, except that now it consisted of silicone instead of rubber.42–44 A 3-year follow-up published in 1993 on six patients who were implanted with the device indicated average results.41,45 An improved design using HP-100 silicon elastomer developed by Steffee et al resulted in the third-generation AcroFlex artificial disk.46,47 However, after the first 40 implantations, the elastomer developed minor defects in several cases, which were evident in a computed tomographic scan after 1 to 2 years. Similarly, later animal studies also indicated poor maintenance of sagittal and lateral flexion ranges of motion.48 A significant advancement in total disk replacement devices was made with the development of the SB Charité (DePuy Spine) prosthesis, which was designed by Schellnac and Büttner-Jans in 1982 and first implanted by Zippel in 1984. (For more details, see related chapter in this book.) The sliding core of this device consisted of ultra high molecular weight polyethylene (UHMWPE) interposed between metallic end plates. However, there were problems with migration and metal fatigue, which led to a second-generation (SB Charité II) device that featured flat extensions on both sides of the end plates. Although this version was an improvement over the previous device, fatigue fractures still led to early failures. Then a third and current version (SB Charité III; DePuy Spine) of the device was developed in 1987, which featured broader, flat end plates. Numerous studies worldwide since 1987 have indicated encouraging and promising results.49 Among these are Griffith et al in 1994,50 Lemaire et al in 1997,51 Cinotti et al in 1996,52 Zeegers et al in 1999,53 McAfee et al in 2003,54 and David in 2005.55 The SB Charité III is currently the most widely implanted total disk replacement system, with more than 7000 implants worldwide.36,52,56,57
Historical Review of Spinal Arthroplasty
and Dynamic Stabilizations
Overview
Spinal Arthroplasty
Nucleus Replacement
Recent Developments
Total Disk Replacement
Lumbar Total Disk Replacement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Overview
Overview Spinal Arthroplasty
Spinal Arthroplasty Dynamic Stabilization
Dynamic Stabilization Summary
Summary