Fig. 5.1
Potential influence of intestinal microbiota on obesity in humans
The ability to analyse gut microbial communities by non-cultural approaches, especially high-throughput sequencing, has led to a great deal of new information on the diversity and composition of the human colonic microbiota. Phylogenetic approaches based mainly on amplified 16S rRNA genes reveal that the two dominant bacterial phyla detected in faecal samples from healthy individuals are the Gram-negative Bacteroidetes and the Gram-positive Firmicutes, with Proteobacteria, Actinobacteria and Verrucomicrobia also represented. Although there is considerable inter-individual variation at the species level, 50–60 dominant species are present in most individuals [8–10]. It is not yet clear whether inter-individual variation in microbiota composition is continuous or semi-discrete, and evidence suggestive of different ‘enterotypes’ [11] within the human population is currently under debate. Communities dominated either by Prevotella spp. or Bacteroides spp. (both belonging to the Bacteroidetes phylum) have been reported from several large studies, but evidence for a third, Firmicutes-dominated, enterotype originally proposed by Arumugam et al. [11] appears less consistent [12, 13]. It now appears that diet may be a major factor driving such variation [12].
Impact of Diet on the Gut Microbiota
The main energy sources available to bacteria in the large intestine are non-digestible carbohydrates (plant cell wall polysaccharides and resistant starch) and host products, especially mucin. There is clear evidence that gut microbiota composition changes with dietary intake. This can be seen in short-term dietary intervention studies, where the representation of certain groups in the faecal microbiota is reported to increase within a few days of switching to diets high in particular non-digestible carbohydrates such as resistant starch [9, 14]. There is also clear evidence from numerous studies for microbiota changes in response to prebiotics [15–18]. These short-term shifts are however strongly influenced by inter-individual variation, and individual variation apparently remains the main factor determining the overall composition of the microbial community despite the consistent response of specific ‘diet-responsive’ bacterial groups [9]. Broadly-based shifts in the gut microbial community have nevertheless been reported in groups of subjects who differ in habitual, long-term dietary intake. In particular, Wu et al. [12] reported higher proportions of Bacteroidetes in subjects consuming diets high in protein, and higher proportions of Prevotella in those consuming more fibre. A similar shift was seen in a group of African children compared with Italian children whose diets differed in fibre and protein intake [19].
In obese subjects, gut microbiota changes have also been shown to result from weight-loss diets. In obese male volunteers there was a significant decrease in the faecal populations of the Roseburia + E. rectale group of Firmicutes bacteria and in bifidobacteria within 4 weeks following a shift to weight-loss diets with high protein and decreased carbohydrate contents [20, 21]. The cross-over design showed clearly that this change was driven by the diet rather than by weight loss, since it was partially reversed when there was a shift to a second weight-loss diet containing a higher content of total carbohydrate [22]. In an earlier study, Ley et al. [23] reported an increase in % Bacteroidetes in obese subjects following 52-week weight-loss regimes. The initial % Bacteroidetes in these subjects was lower than in most other studies, as discussed below.
Evidence for Changes in Microbiota Composition in Obese Humans
An early report, based on sequencing of amplified 16S rRNA genes, indicated a much higher ratio of Firmicutes to Bacteroidetes in faecal samples from 12 obese humans than in two lean controls [23]. This appeared consistent with the higher Firmicutes:Bacteroidetes ratio seen in genetically obese mice compared with lean mice, leading to the proposal that the ratio of these two phyla within the gut microbiota might be a causative factor in obesity [1]. Subsequent studies using FISH microscopy however either did not detect a change in % Bacteroidetes with BMI [22] or indicated a slightly increased % Bacteroidetes [24] in obese subjects. Numerous studies have now been performed using quantitative PCR, high-throughput sequencing of 16S rRNA genes or metagenome sequencing to analyse the faecal microbiota in obese subjects, leading to the conclusion that there is no consistent difference between lean, normal weight and obese subjects at the bacterial phylum level [4, 9, 25–27]. Some differences are however apparent at the genus and species levels [28] including between MZ twins who were discordant for BMI [29]. Given the impact of diet on microbiota composition at this level, discussed above, it seems likely that such differences will at least partially reflect differences on dietary intake between obese and normal-weight individuals. Yokota et al. [30] recently suggested that increased secretion of bile acids may contribute to alterations of the microbiota on high fat diets due to the antimicrobial activity of secondary bile acids. They demonstrated microbiota changes in line with those seen in several studies on high fat diets (i.e. an increase in Firmicutes at the expense of Bacteroidetes) after feeding rats increasing levels of cholic acid. There is also increasing evidence that type 2 diabetics show altered microbiota profiles when compared with healthy subjects, with a decreased representation of certain groups of Firmicutes and of bifidobacteria [31–34]. The increased incidence of metabolic syndrome and type 2 diabetes in obese subjects is therefore an important confounding factor when interpreting microbiota changes in the obese.
Recent work using metagenomic sequencing has shown that microbiota profiles in obese subjects can be distinguished as being of low (LGC), or high (HGC) gene count, reflecting high and low species diversity [28]. The LGC type tends to be dominated by the Gram-negative Bacteroides and may correspond to one of the ‘enterotypes’ proposed by Arumugam et al. [11]. Obese or overweight subjects, showing the LGC profile had significantly higher insulin resistance and fasting triglyceride levels, indicative of metabolic syndrome, compared with HGC individuals [28]. Moreover, obese LGC individuals showed more rapid past weight gain on average than obese HGC individuals. In a companion study, a 12-week intervention on weight-loss diets increased the gene count in the LGC group, while improving symptoms associated with metabolic syndrome in both groups [35]. The simplest interpretation of these findings is that gut microbiota composition in these subjects is largely driven by their dietary intake, although, conversely, consequences of changes in host physiology could also influence microbiota composition. A diet that is low in fibre and high in digestible carbohydrates, especially simple sugars, might account for the LGC profile (found in both obese and lean individuals) while at the same time promoting the development of metabolic syndrome.
In human studies it is usually not possible to distinguish between microbiota changes that are consequences of changes in diet and/or host physiology from any that might be contributing factors in obesity, adiposity and inflammation. However there is intriguing evidence, mainly from animal studies to suggest that individual bacteria could have more significant roles in influencing host nutrition, physiology and behaviour. A number of studies have shown that transfer of gut microbiota from obese humans, compared with non-obese donors, to germ-free mice results in increased weight gain and adiposity in the colonised mice. Most recently, this result has been reported for obese human twin pairs that were discordant for BMI with the microbiota from the obese twin promoting adiposity and weight gain when transferred into germ-free mice to a greater extent than the microbiota from the relatively lean twin [36]. Diet is likely to have driven the separation in the microbiota composition between the members of each twin pair, but the transfer experiments suggest that this altered composition is also contributing to adiposity and weight gain. Such effects require mechanistic explanations and some of the possibilities are considered below.
Potential for Microbiota Composition to Influence Energy Recovery from the Diet
The gut microbial community in the large intestine contributes to the overall ‘energy harvest’ from the diet by fermenting components that remain undigested by host enzymes in the small intestine. Short-chain fatty acids produced by microbial fermentation are efficiently transported across the gut wall and used as energy sources, with butyrate being preferentially utilised by the gut epithelium. The over-riding factor that determines how much energy is delivered via microbial fermentation is the non-digestible carbohydrate (fibre) content of the diet, together with gut transit, which is of course influenced by fibre content [5, 37]. More rapid whole gut transit may lead to a greater fraction of dietary intake failing to be digested in the upper GI tract, thus increasing the substrate available for fermentation in the large intestine [38]. On the other hand, rapid transit also tends to decrease the extent of fibre degradation and the efficiency of SCFA absorption [37]. Faecal SCFA concentrations are reported to be higher in obese subjects [24, 39, 40] which seems most likely to reflect higher dietary intake.
The potential factor that has attracted most speculation is the species composition of the gut microbiota [25, 41]. It is therefore worth considering in more detail the mechanisms by which changes in microbiota composition might affect energy recovery from the diet by considering the following questions.
Does Microbiota Composition Influence the Rate and Extent of Substrate Fermentation in the Colon?
If certain ‘keystone’ species were required to initiate degradation of recalcitrant substrates, then their absence from the microbiota could have a major impact on the release of energy from dietary residue. An example of this phenomenon comes from the finding that, among 14 obese human volunteers, ingested RS3 starch remained largely unfermented only in two individuals who lacked ruminococci in their faeces [9]. Relatives of R. bromii appear to be particularly potent degraders of this type of starch by comparison with other amylolytic species [42]. It is currently unclear how common such deficiencies in ‘keystone species’ are within the general population, but their consequence would be to reduce energy gain.
Does Microbiota Composition Influence the Stoichiometry of Fermentation in the Gut?
In vitro experiments show clearly that perturbation of the microbial community composition, e.g. resulting from a pH change, can result in major shifts in the ratios of the major fermentation products [43]. While acetate, propionate and butyrate all supply energy to the host, they are utilised by different tissues and have different physiological consequences, as discussed further below. Deficiencies in butyrate-producing bacteria (which belong to the Firmicutes within the human colonic community) have now been reported in several disease states, including type 2 diabetes [34], and overall decreases in these bacteria are known to result in decreased butyrate production [20, 43].
Hydrogen utilisation plays a central role in anaerobic metabolism, and the consequences of variation in hydrogen utilising microbes have been the subject of much speculation. Methanogenic archaea occur in high numbers in approximately half of the population; some reports indicate that they are increased in obese subjects [40, 44] while others suggest the contrary [24]. It is possible that in the absence of methanogenesis more carbon would be diverted into SCFA and therefore to the host (Table 5.1); indeed this is one of the goals of attempting to inhibit methanogenesis in the rumen [45]. In the absence of inhibition, however, the equivalent amount of carbon may simply be released as CO2 when methanogen populations are low. Another important group of hydrogen utilisers are acetogens, which have the ability to convert H2 and CO2 (or formate) to acetate. This introduces an additional non-dietary source of acetate carbon [46] but the contribution and degree of variability of this route for acetate synthesis in the human colon has not been fully established.
Table 5.1
Possible impacts of methanogenic archaea and methanogenesis upon ‘energy harvest’ from the diet
Consequence of methanogenic activity | Consequence for energy harvest |
|---|---|
1. Net loss of carbon as CH4 | Decreased? [BUT without methanogenesis equivalent C may simply be lost as CO2] |
2. Increased efficiency of energy metabolism by H2-producing bacteria | Increased? [BUT same increase will apply to acetogenic bacteria + H2 producers?] |
3. Methanogenic activity correlates with slow gut transit | Increased? Fibre degradation and SCFA absorption more efficient at slower transit times? |
Another effect of hydrogen utilisation is to increase the growth efficiency of hydrogen-producing, substrate-degrading species [47]. As this is predicted to occur with both methanogens and acetogens it would be assumed to apply to any ‘normal’ gut microbial community, although there is some intriguing evidence that cellulolytic ruminococci may be dependent on the presence of methanogens within the microbiota [48]. If degradation of some dietary carbohydrates was increased in the presence of methanogens, this might tend to increase ‘energy harvest’ (Table 5.1).
Does Microbiota Composition Affect the Uptake of SCFA by the Gut Epithelium?
As already noted, gut transit is thought to have an important influence of SCFA uptake. Conversely, SCFA are themselves known to affect gut motility and transit via interactions with receptors that influence gut hormones, although these effects may differ in different regions of the intestine. This creates complex feedback loops whereby microbiota composition may influence absorption of fermentation products by the host. There is also intriguing evidence that methane may slow gut transit [49]; methanogens appear to be associated with slow gut transit [50] but whether this is because of their slow growth rate or their impact on gut motility is not known.
Potential for Microbiota to Influence Energy Expenditure and Adiposity
The intestinal microbiota influences host physiology beyond their direct actions in the gut (Fig. 5.2). Several possible routes of cross-talk exist between the microbes and host tissues, encompassing metabolic, immunological, endocrine and neural pathways [3, 51, 52], and the exact mechanisms of interaction are currently under extensive research. Another factor that has to be taken into consideration is the role host genetics play in determining the response to obesogenic diets as well as the composition of the microbiota. High heritability of gene-by-diet interactions has recently been demonstrated in a genome-wide association study in different mouse strains on a high fat/high sugar diet [53]. A parallel investigation of the gut microbiota revealed significant phylum-level shifts in response to diet across different genetic backgrounds, however, effects of the genetic background on the composition and plasticity of the microbiota were also evident. Only one of the genetic loci found to be associated with body fat, which include three amylase genes, was found to be associated with significant changes in microbiota composition, namely an enrichment of Enterobacteriaceae within the phylum Proteobacteria. In addition, three specific microbiota phylotypes showed a modest correlation with obesity traits. Intriguingly, Akkermansia displayed a negative correlation with body fat percentage despite the fact that this genus showed the strongest overall enrichment on the high fat/high sugar diet [53].
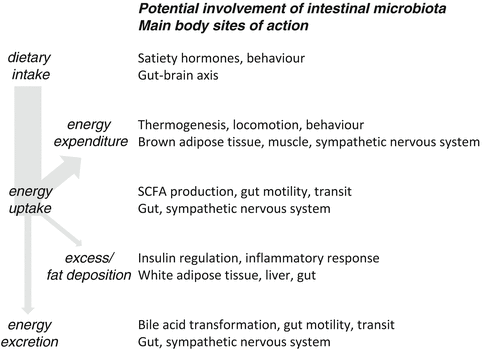

Fig. 5.2
Potential influences of intestinal microbiota on energy balance in humans
Some studies have found that energy balance could be profoundly influenced in animal models by the introduction of a single bacterial species. Administration of a purified probiotic strain of Lactobacillus reuteri led to the prevention of weight gain without significantly affecting the existing microbiota or calorie consumption in mice on a Western diet. The underlying effect appeared to be a modulation of the immune system towards a more anti-inflammatory tone, and the phenotype was transferable to naïve hosts via purified CD4+ T cells from animals consuming the probiotic [54].
Separately, it has been reported that Akkermansia muciniphila (which comprises 3–5 % of the colonic microbiota in healthy adults) abundance correlates inversely with body weight. This bacterium has a specialist role and derives its carbon and energy from the mucus layer lining the intestinal tract. In contrast to Parks et al. [53] who found an increase of Akkermansia on a high fat/high sugar diet (see above), Everard et al. [55] demonstrated that populations of this organism are diminished on high fat diets, which resulted in a reduction in the thickness of the mucus layer. Moreover, re-introduction of A. muciniphila by gavage to mice fed a high fat diet reduced body weight and improved body composition without changes in food intake. It also restored the mucus layer, decreased circulating lipopolysaccharide (LPS) levels and increased glucose tolerance compared to control animals gavaged with either PBS or killed cells [55]. Both studies found that the reduction in weight gain and body fat was achieved without a significant reduction in food intake, indicating that energy balance regulation was influenced via other factors, such as locomotor activity and heat production.
A direct link between obesity, glucose metabolism and low-grade inflammation has previously been demonstrated by subcutaneous administration of LPS, which led to insulin resistance and fat mass development in mice [56]. An intact gut barrier function is crucial in preventing LPS from crossing from the gut into the systemic circulation (increased plasma LPS levels have been termed metabolic endotoxemia), and the gut microbiota may influence gut permeability via actions on the mucus layer or regulatory effects on epithelial cells (e.g. tight junction protein expression) [57]. Bacterial signalling appears to involve the endocannabinoid system, endogenous bioactive lipids that regulate barrier function, as well as the enteroendocrine peptide glucagon-like peptide-2 [55].
Inflammation may be mediated by several bacterial products such as lipopeptides, LPS and flagellins that act as ligands for toll-like receptors (TLRs) 2, 4 and 5, whilst other TLRs detect nucleic acid motifs. In most cell types detection of these bacterial ligands evokes a potent inflammatory response inducing myeloid-differentiation factor 88 (MyD88) and NF-kappa B which results in a broad array of pro-inflammatory chemokines and cytokines. By contrast, recognition of these bacterial moieties by intestinal epithelial cells has been reported to lead to enhancement of barrier function, and epithelial repair rather than overt inflammatory responses.
LPS is continuously released in the intestinal tract as a consequence of bacterial cell lysis and serum LPS was shown to be 76 % higher in type 2 diabetics compared to the control cohort and consumption of a high fat meal resulted in a 50 % higher endotoxin level [58]. High fat diets can increase absorption of LPS present in the cell walls of Gram-negative bacteria either by incorporation into chylomicrons or by increasing intestinal permeability [56]. LPS is a potent inflammatory mediator that signals in a TLR4-dependent manner and infusion of LPS can increase weight gain, adiposity, insulin resistance and liver triglycerides. Separately deletion of TLR5 in mice, which senses bacterial flagellin, results in an alteration in the composition of the gut microbiota and also to features of metabolic syndrome including insulin resistance, increased adiposity and blood pressure and increased cholesterol levels [59].
An important role of inflammation in the development of obesity is in line with the notion that germ-free animals are resistant to diet-induced obesity [60]. However, Fleissner et al. [61] demonstrated that this effect is dependent on the specific dietary ingredients of high fat diets and that germ-free mice are not generally protected against obesity by comparing different types of high-fat diet with equal macronutrient content. Furthermore, it has recently been shown that, in contrast to germ-free mice, germ-free rats did not exhibit decreased adiposity compared to their conventional counterparts, and alterations in host lipid metabolism differed between rats and mice [62]. Therefore, differences in host metabolism as well as morphological and physiological alterations of germ-free animals compared to conventional animals require careful consideration in the assessment of microbiota-mediated effects on adipogenesis.
Recently Upadhyay et al. [63




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





