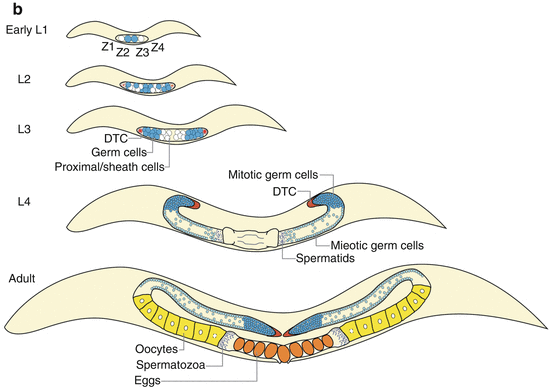
Fig. 3.1
Germline organization in C. elegans. (a) Germline specification relies on maternally inherited germ granules, called P granules. P granules contain RNA and proteins required for germline determination and are progressively segregated into a series of P blastomeres (highlighted as turquoise cells) during embryonic development. P4 will eventually divide into two PGCs (Z2 and Z3, turquoise) at the ~100-cell stage. (b) In postembryonic development, the Z2 and Z3 PGCs will continue to proliferate and comprise the future germ cell population after hatch, which are supported by the DTCs (red) and sheath cells developed from Z1 and Z4 SGPs (white). The DTCs locate at the distal ends of gonad arms, acting as the niche for GSC maintenance and proliferation. Germ cells proliferate mitotically (turquoise) in the distal region; when progressing into the meiotic region, germ cells become meiotic (light blue) and undergo sperm (purple dot) differentiation at the L4 stage and oogenesis (yellow) later during adulthood. The matured oocyte goes through spermatheca and becomes self-fertilized egg (orange) in the uterus. (c) The DTC projects processes and surrounds the distal end of the gonad arm. Those germ cells adjacent to the DTC remain mitotic; otherwise, they turn into meiotic differentiation program. This is controlled via LAG-2/GLP-1 Notch-like signaling pathway. In brief, the DTC-expressed LAG-2 triggers the activation of its receptor GLP-1 and downstream FEB-1/2 signaling in GSCs. FEB-1/2 maintain mitotic proliferation fate by promoting mitosis-specific mRNA translation via inhibiting GLD-1/NOS-3 and by suppressing meiosis-specific mRNA translation via inhibiting GLD-2/3. When germ cells progress toward the proximal end, they lose the DTC signaling; thus, GLD-1/NOS-3 and GLD-2/3 take the lead to switch on meiotic program. Abbreviation: PGC primordial germ cell, SGP somatic gonad precursors, DTC distal tip cell, GSC germline stem cell
Germline proliferation, survival, and differentiation continue through L1 to L3 larval stages, followed by gametogenesis at L4 and early adulthood (Fig. 3.1b) (Hubbard and Greenstein 2005). When nutritional condition is favorable, PGCs undergo MES-mediated chromatin modification to activate proliferation genes, divide symmetrically to expand syncytial GSC population, and ultimately give rise to germline tissues. Meanwhile, each SGP differentiates asymmetrically into five proximal cells and one distal tip cell (DTC). The proximal cells eventually turn into sheath cells, forming single layer intimately surrounding the germ cells. The DTC, at the distal end of each germline arm, forms processes that stretch out proximally along germline surface and functions as a niche to maintain the adjacent GSCs (Fig. 3.1c). During the L4 larval stage, the polarity of the germline forms. As new cells continue proliferating at the distal end, the other germ cells are pushed toward the proximal side and farther away from the DTC. Only GSCs and early germ cells at the distal end remain mitotic. In contrast, those germ cells progressing to the proximal end switch fate from mitotic proliferation to meiotic differentiation and undergo spermatogenesis during the L4 stage, followed by oogenesis starting at adulthood (Fig. 3.1b). In adulthood, oogenesis can continue throughout the whole reproductive period until sperm exhaustion.
3.2.2 Molecular Mechanism Controlling GSC Maintenance and Activity
In C. elegans, GSCs are located at the most distal end of the germline. The somatic DTC extends thin cytoplasmic processes that encircle the most distal end germ cells and serves as a niche for adjacent GSCs (Fig. 3.1c) (Li and Xie 2005; Xie 2008). When DTC is killed by laser ablation, the germline mitotic region diminishes (Kimble and White 1981). Conversely, DTC relocation results in the development of ectopic proliferating germ cells at the corresponding position, and DTC duplication leads to doubling of germ cell pools (Kidd et al. 2005; Kipreos et al. 2000; Lam et al. 2006). The DTC promotes mitotic proliferation of GSCs via GLP-1/Notch signaling. LAG-2, a notch-like signaling ligand, is expressed in the DTC and activates its receptor GLP-1, located on the surface of GSCs (Fig. 3.1c) (Crittenden et al. 1994; Fitzgerald and Greenwald 1995; Henderson et al. 1994). The activation of GLP-1/Notch signaling is necessary and sufficient for GSC maintenance and proliferation. In the loss-of-function mutants of glp–1, all of the germ cells progress into meiotic differentiation, leading to a complete loss of the GSC population (Fitzgerald and Greenwald 1995). Conversely, gain-of-function mutations of glp–1 promote GSC overproliferation and the formation of germline tumor (Berry et al. 1997).
In response to the activation of GLP-1/Notch signaling, a battery of downstream RNA regulators cooperate to promote GSC self-renewal and prevent cell differentiation (Fig. 3.1c) (Byrd and Kimble 2009; Kimble and Crittenden 2005). In brief, GLP-1 can transcriptionally activate fbf–2 (along with other unidentified targets) (Lamont et al. 2004). FBF-2 is a pumilio-like RNA-binding translational repressor that can work together with another pumilio-like protein FBF-1 to repress the expression of gld–1 and gld–3 via binding to their 3′-untranslated regions (UTRs) (Crittenden et al. 2002; Eckmann et al. 2004). GLD-1 together with NOS-3 and GLD-3 together with GLD-2 form two parallel regulatory branches to control meiotic entry in the germline. In one branch, GLD-1 functions as a translational repressor that acts through regulatory elements present in the 3′-UTR of mitosis-promoting genes and represses their expression posttranscriptionally. NOS-3, a Nanos family of RNA-binding proteins, physically interacts with FBF-1 and promotes GLD-1 accumulation (Hansen et al. 2004). In the other branch, GLD-2, a cytoplasmic poly(A) polymerase and translational activator, activates meiosis-promoting genes while GLD-3, a member of the bicaudal-C family of RNA-binding protein, enhances the poly(A) polymerase activity of GLD-2 and antagonizes the FBF-mediated repression (Eckmann et al. 2002, 2004; Wang et al. 2002). Together, GLP-1/Notch signaling and FBF-GLD proteins constitute the control hub for GSC maintenance in adults (Fig. 3.1c).
3.3 GSC Arrest Promotes Longevity in C. elegans: Trade-Off Between Reproduction and Longevity?
As mentioned in the introduction, we now know that the aging process is under the control of various regulatory pathways (Blagosklonny et al. 2010; Kenyon 2010b). Altered activity of those pathways by genetic or pharmacological methods can effectively influence organism lifespan. The first of such pathways was characterized in C. elegans and named the insulin/IGF-1 signaling (IIS) pathway. Mutations of age–1 or daf–2, the worm homolog of phosphoinositide 3-kinase (PI3K) or insulin/IGF-1 receptor, respectively, were shown to increase C. elegans adult lifespan by two-folds (Friedman and Johnson 1988; Kimura et al. 1997; Samuelson et al. 2007). IIS activation is transduced through PDK-1 (PIP3-dependent kinase 1) and AKT-1/2 (AKT/protein kinase B) and inhibits the transcriptional activity of the FOXO forkhead transcription factor, DAF-16. Loss-of-function mutations of pdk–1 or akt-1/2 increase C. elegans lifespan (Paradis et al. 1999; Paradis and Ruvkun 1998) while the increased lifespan associated with daf–2 or age–1 mutants is suppressed by mutations in daf–16 (Ogg et al. 1997). Following C. elegans studies, the IIS pathway has been shown to regulate lifespan also in yeast, fruit flies, and mice (Longo et al. 2005). In human, several insulin receptor and IGF-1 receptor variants are linked to extremely long life in Ashkenazi Jewish centenarian and Japanese centenarian, respectively (Kojima et al. 2004; Suh et al. 2008). Furthermore, a number of AKT and FOXO3 variants have been implicated to be responsible for human exceptional longevity, as shown in multiple independent genetic association studies (Anselmi et al. 2009; Flachsbart et al. 2009; Pawlikowska et al. 2009; Willcox et al. 2008).
To date, the IIS pathway has been well recognized as a remarkable conserved mechanism to modulate the aging process across species. However, when first identified, its life-extending effect was suspected to be a result of fertility trade-off. Could it be possible that the daf–2 and age–1 mutants live longer simply because of their comprised reproductive abilities and consequent allocation of energy resources from reproduction to somatic maintenance? Several lines of evidences do not agree with this assumption. First of all, sterility alone, either by physical removal of whole gonad or using non-reproducing mutants, is not sufficient to ensure longevity (Kenyon et al. 1993). Secondly, fer–15 rescue can restore normal fertility in the age-1 mutants but has no effect on the lifespan extension (Johnson et al. 1993). In addition, the daf–2 mutants remain long lived at 15 °C, where the animals have similar reproductive ability as wild type (Tissenbaum and Ruvkun 1998). Last but not least, knockdown of daf–2 by RNAi only during adulthood extends lifespan without affecting reproduction while daf–2 inactivation during development reduces fertility with no lifespan extension (Dillin et al. 2002). Therefore, decreased fertility in the daf–2 mutants is likely due to its requirement during development, but not related to its longevity effects at adulthood.
So, what is the relationship between reproduction and longevity, if there is no simple trade-off between them? The merging view is that the reproductive system can produce regulatory signals that actively coordinate the metabolic state of the organism toward reproduction or toward survival. In the following sections, we will focus on the mechanistic details of how reproductive signaling might regulate lifespan in C. elegans.
3.4 Germline, GSCs, and Longevity
The first line of evidence that the reproductive system produces signals to regulate lifespan came from the classic experiments by the Kenyon laboratory (Hsin and Kenyon 1999). They showed that removal of the germline precursors Z2/3 PGCs by laser microsurgery results in germline-less animals that live 60 % longer than the mock control, while additional removal of the somatic precursors Z1/4 SGPs, which eliminates somatic gonad surrounding and supporting the germ cells, abrogates this lifespan extension (Hsin and Kenyon 1999). These suggest that the longevity effect conferred by germline ablation is not a simple consequence of sterility, but rather arises from certain regulatory signals. Removal of somatic gonad may antagonize those longevity-promoting signals (Hsin and Kenyon 1999). In addition, the longevity phenotype in the germline-less animals resulting from physical ablation can also be recapitulated using genetic mutants. Loss-of-function mutations in mes–1 that have no germ cells can live twofold longer, and the glp–1 mutants that arrest germ cell proliferation can live 1.5-fold longer (Arantes-Oliveira et al. 2002). In both studies of mes–1 and glp–1, the presence of somatic gonad is required for the longevity effects.
In the adult germline, different stages of proliferative and differentiated germ cells, including GSCs, mitotic germ cells, meiotic germ cells, oocytes, and sperms, are present at one time (Fig. 3.1b). Which of those specific germ cell groups contribute to the lifespan regulation? Surprisingly, several different sperm-deficient (fem–1, fog–1, fog–2, or fog–3) or oocyte-deficient (daz–1) mutants all exhibit normal lifespan without extension (Arantes-Oliveira et al. 2002). It indicates the negligible functions of gametogenesis in the regulation of organism lifespan. This is particularly unexpected for the oocyte-deficient mutants, given that vast energy is invested during oocyte formation. These observations further support that the lifespan extension conferred by germline deficiency is not a simple passive result of energy reallocation. On the other hand, both GLP-1/Notch signaling and FBF-GLD proteins are required for GSC maintenance and proliferation. Interestingly, loss-of-function mutations of glp–1 that arrest GSCs promote longevity, while glp–1 gain-of-function mutations or gld–1 loss-of-function mutations that cause GSC overproliferation in contrast shorten lifespan (Arantes-Oliveira et al. 2002). These observations reveal the crucial function of GCSs in regulating longevity. Furthermore, in the temperature-sensitive glp–1 mutants, GSC arresting upon temperature shift can always lead to increased longevity, even at day-1 adulthood when the whole germline development is completely accomplished (Arantes-Oliveira et al. 2002). Thus, GSC arrest itself is sufficient to promote longevity, and the lifespan regulatory signals could directly associate with the presence of GSCs.
Importantly, this GSC longevity regulatory mechanism is evolutionarily conserved. In Drosophila, adult flies lacking GSCs live 50 % longer than the controls (Flatt et al. 2008). In mice, ovary transplantation from young females increases the lifespan of older female recipients (Cargill et al. 2003), suggesting that unknown lifespan-enhancing endocrine signals are produced from mammalian ovary tissues.
3.5 Signaling Pathways Regulating Longevity in Response to Gonadal Signals
3.5.1 Steroid Hormone Control from the Reproductive System
Reproductive tissues are crucial functional parts of the endocrine system. Steroid hormones synthesized and released from the reproductive system can systemically coordinate the whole body physiology. Consistently, steroidal signaling has been implicated as a key factor contributing to the regulation of GSC longevity (Fig. 3.2). This includes the bile acid-like steroids (dafachronic acids (DA)), its receptor DAF-12, and several enzymes involved in DA synthesis, such as DAF-36/Rieske-like oxygenase, DHS-16/3-hydroxysteroid dehydrogenase, and DAF-9/cytochrome P450 (Lee and Schroeder 2012). Loss-of-function mutations of daf–12, daf–36, dhs-16, or daf–9 completely abrogate the longevity in the germline-less animals conferred by the glp–1 mutations or Z2/3 laser ablation (Beckstead and Thummel 2006; Gerisch et al. 2001; Wollam et al. 2012). Moreover, DA supplementation restores longevity in the daf–9;glp–1, dhs–16;glp–1, and daf–36;glp–1 double mutants back to that of glp–1 but has no effect in extending the shortened lifespan of daf–12;glp–1 (Gerisch et al. 2007; Wollam et al. 2012). Thus, DA biosynthesis and its transcriptional activation of DAF-12 are both required in promoting longevity of the germline-less animals.
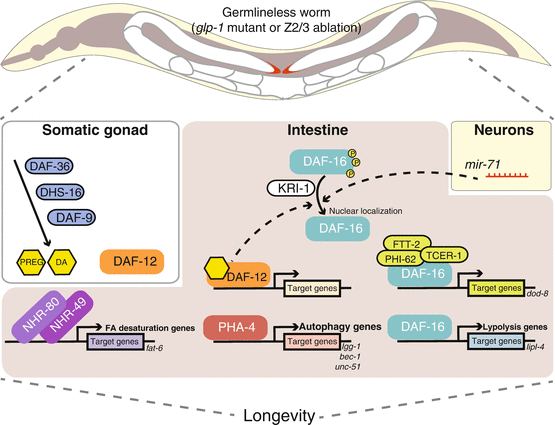

Fig. 3.2
Longevity regulation in germline-deficient C. elegans. Germ cell loss works in concert with somatic gonad, intestine, and neuronal cells to regulate lifespan extension. In somatic gonad, steroid synthesis via DAF-36, DHS-16, and DAF-9 generates hormone ligands, like DA and PREG, for DAF-12 activation. DAF-12 promotes longevity via regulating downstream target gene expression and via assisting FOXO/DAF-16 nuclear localization in the intestine. DAF-16 activation also requires KRI-1 and neuronal mir–71 activity. Once DAF-16 enters nucleus, it activates assorted downstream targets through forming distinct complexes with different factors. When DAF-16 interacts with TCER-1, PHI-62, and FTT-2, it is known to regulate the gene dod–8. DAF-16 also plays a central role in regulating lipl–4 and other lipolysis genes. With the aid of lipl–4, FOXA/PHA-4 regulates autophagy gene expression, e.g., lgg–1, bec–1, and unc–51. On the other hand, NHR-80 and NHR-49 activate the expression of SCD, including fat–6, to alter lipid composition by increasing oleic acid synthesis. These subsets of regulatory modules do not work self-sufficiently; instead, they belong to a closely linked network and may have partial dependency on others. These findings also suggest there is cross-tissue endocrine signaling to communicate the whole organism in the germline-depleted scenario and to achieve lifespan extension. Abbreviation: DA dafachronic acid, PREG pregnenolone, SCD stearoyl-CoA-Δ9-desaturases
As mentioned above, an intact somatic gonad is required for the longevity of the germline-less animals, suggesting somatic gonad as a source of life-extending signals. Notably, DA supplementation can restore longevity of the whole gonad-deficient worms lacking both germline and somatic gonad, which requires the presence of daf–12 (Gerisch et al. 2007). In addition, DAF-12 transcriptional activities are stimulated by germline loss, but further removal of somatic gonad diminishes this induction, which can be rescued by supplying DA exogenously (Yamawaki et al. 2010). These indicate that DA biosynthesis and DAF-12 signaling are both involved in mediating life-extending signals from the somatic gonad. In wild-type animals, those signals may be hindered by the presence of germline. Besides DA, C. elegans also contain several other hormonal steroids that are present in humans, including pregnenolone (3β-hydroxy-pregn-5-en-20-one; PREG) and other pregnane and androstane derivatives (Broue et al. 2007). Some of those steroids are also involved in the regulation of longevity in the germline-less animals. For example, in the glp–1 mutants, PREG levels are elevated in a DAF-9-dependent fashion. Moreover, PREG supplementation restores longevity in the daf–9;glp–1 double mutants, but fails to do so in the daf–12;glp–1 mutants, suggesting the involvement of PREG in mediating gonadal longevity signals (Broue et al. 2007). However, neither DA nor PREG supplementation is sufficient to extend lifespan in wild type or further increase longevity of germline-deficient animals. Therefore, there must be also other signaling components mediating the longevity effects conferred by germline loss.
3.5.2 Regulation of Intestinal FOXO Activity Upon Germline Loss
The FOXO forkhead transcription factor DAF-16 serves as another central regulator of longevity by germline deficiency (Fig. 3.2). Null mutation of daf–16 completely abolishes the longevity of the germline-ablated animals (Hsin and Kenyon 1999) and the glp–1 germline-defective mutants (Arantes-Oliveira et al. 2002; Berman and Kenyon 2006). Despite ubiquitous distribution in all worm tissues, DAF-16 activities in the intestine may be particularly critical to mediate the longevity effect brought about by germline loss. Upon laser microsurgery of germline precursor cells or in the glp–1 germline-deficient mutants, DAF-16 is translocalized to the nucleus in intestinal cells during the first day of adulthood, but its subcellular localization remains unchanged in other tissues (Berman and Kenyon 2006; Lin et al. 2001). Similar to mammalian FOXO transcription factors, translocation of DAF-16 from the cytoplasm to the nucleus triggers its activation, and three conserved AKT phosphorylation sites are required for its cytoplasmic retention. Substitution of those three consensus sites with alanines generates a constitutively nuclear DAF-16 protein (referred to as DAF-16 AM) (Lin et al. 2001). Importantly, expression of this mutated form of DAF-16 AM within the intestine fully restores the longevity of the glp–1;daf–16 double mutants, showing the functional significance of DAF-16 nuclear localization in this tissue (Berman and Kenyon 2006). The worm intestine is not only a digestive organ, but also a key metabolic and endocrine organ to store lipid and release humoral signals to the rest of the organism. Thus, activation of DAF-16 in the intestine by germline signals may generate secondary effects and hence have global impacts on organism longevity.
Multiple factors genetically act upstream of DAF-16 in response to germline loss. First of all, DA/DAF-12 signaling assists DAF-16 nuclear localization in the germline-less animals. Loss-of-function mutations of either daf–9, daf–36, dhs–16, or daf–12 substantially compromise the nuclear translocation of DAF-16 in the intestine, and DA supplementation restores DAF-16 nuclear localization in the glp-1 double mutants with those DA biosynthesis genes (Gerisch et al. 2007; Libina et al. 2003; Wollam et al. 2012). Noteworthily, expression of constitutively nuclear localized DAF-16 AM fails to restore longevity in glp–1; daf–16; daf–12 mutants (Berman and Kenyon 2006). It suggests that DAF-12 may not only facilitate DAF-16 nuclear translocalization, but also aid its transcriptional activation or have other unidentified downstream pathways. Secondly, scientists also apply unbiased genetic screens to search for other components involved in the germline longevity signaling. kri–1 (Berman and Kenyon 2006) and tcer–1 (Ghazi et al. 2009) are two genes identified in large-scale RNAi screens that remarkably suppress the longevity of the glp–1 mutants but have no effect on wild-type lifespan. KRI-1, a worm homolog of the human KRIT/CCM1 protein, is expressed in the intestine and is required for the intestinal nuclear localization of DAF-16 in the glp–1 mutants (Berman and Kenyon 2006). In the presence of DAF-16 AM, kri–1 loss-of-function mutations cannot abrogate the longevity of the glp–1 mutants (Berman and Kenyon 2006). Thus, KRI-1 is likely a downstream intestinal factor of the germline longevity signaling pathway, and its primary function is to promote DAF-16 nuclear localization in this tissue. As a transcription elongation factor, TCER-1 works together with DAF-16 in regulating the expression of specific target genes in response to germline depletion. tcer–1 is specifically upregulated in the nuclei of intestine and neurons in the germline-less animals, and its intestinal upregulation is dependent on kri–1, but not daf–12 (Ghazi et al. 2009). Although loss-of-function mutations of tcer–1 do not affect DAF-16 nuclear localization in the germline-less animals, they reduce the induction of several DAF-16 target genes and abrogate the longevity (Ghazi et al. 2009). Conversely, TCER-1 overexpression significantly triggers specific DAF-16 target gene expressions, along with lifespan extension by 15 %, which is dependent on DAF-16 activity (Ghazi et al. 2009). The mammalian homolog of TCER-1, TCERG-1, associates with RNA polymerase II and regulates transcription elongation and pre-mRNA splicing (Carty et al. 2000; Goldstrohm et al. 2001; Lin et al. 2004; Pearson et al. 2008; Smith et al. 2004; Sune and Garcia-Blanco 1999). A plausible model is that germline removal triggers the formation of specialized transcriptional complexes including TCER-1, DAF-16, and other unidentified factors, which modulate the expression of a specific subset of genes that are crucial for organism longevity. This is supported by the findings that TCER-1 and DAF-16 physically interact with FTT-2 (14-3-3 protein) and PHI-62 (RNA-binding protein) to regulate dod–8 (putative steroid dehydrogenase) expression, and both ftt–2 and phi–62 are required for the longevity in germline-less animals (Li et al. 2007; McCormick et al. 2012). However, the expression of sod–3 (Mn++ superoxide dismutase), another daf–16 downstream target, does not require ftt–2 nor phi–62 (McCormick et al. 2012). This reveals that DAF-16 may form a variety of complexes with different factors to exert diverse regulatory functions on multiple subsets of downstream genes and together create a network that promotes organism longevity (Fig. 3.2).
Interestingly, recent studies have also implicated neuronal microRNA mir–71 in the regulation of DAF-16 nuclear localization and longevity in the germline-less animals (Boulias and Horvitz 2012). In a large screen of microRNA loss-of-function mutants, mir–71 loss-of-function was identified to shorten wild-type lifespan by 40 %. More importantly, loss of mir–71 fully abrogates the longevity in the glp–1 mutants or the germline-ablated animals and also blocks intestinal nuclear localization of DAF-16 and its target gene expressions in those germline-less animals (Boulias and Horvitz 2012). Conversely, mir–71 overexpression modestly increases lifespan in wild type and further enhances the glp–1 longevity. This increased longevity fully requires DAF-16 and TCER-1 activities within the intestine but is not affected by DAF-12. Although broadly expressed in multiple tissues, mir–71 activity within neurons is particularly important to mediate the longevity effects by germline loss. Thus, mir–71 acts in a cell nonautonomous manner to regulate DAF-16 activity in the intestine. A complex crosstalk among the reproductive system, neurons, and intestine systemically regulates the organism aging process (Fig. 3.2).
3.5.3 Interaction with the IIS Pathway in the Regulation of Longevity
As mentioned above, the IIS pathway is the first identified, the best characterized, and the most conserved longevity mechanism to date. Whether and how does the germline longevity signaling interact with the IIS pathway? So far, the evidences suggest that these two mechanisms largely work independently (Kenyon 2010a; Panowski and Dillin 2009). First, germline deficiency and reduced daf–2 activity have synergistic effects genetically in promoting longevity. Ablation of germ cells in the daf–2 loss-of-function mutants further enhances already increased lifespan in the mutants (Hsin and Kenyon 1999). Second, DAF-16 responds to germline loss signals differently from reduced IIS. Removal of germline causes DAF-16 localization in intestinal nuclei at adulthood, while daf–2 loss-of-function mutants display DAF-16 nuclear accumulation in all somatic cells throughout life (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001). Third, as discussed above, multiple factors are required to promote longevity in the germline-less animals, including daf




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree