and Veronica Tomasello2
(1)
Department of Plastic Surgery and Burns, University Hospital Vall d’Hebron, Barcelona, Spain
(2)
Cannizzaro Hospital, Catania, Italy
Abstract
Postoperative care of composite tissue allotransplantation patients has significant differences when compared to other solid organ transplantation procedures. In general terms, the management of these types of patients is less complex than solid organ transplantation; however, some specific issues complicate it, including immunological barriers, antigen load of composite tissues, surgical stress, and bleeding and fluid management.
Keywords
Postoperative evaluationComplicationsMedicationsPostoperative care of composite tissue allotransplantation patients has significant differences when compared to other solid organ transplantation procedures. In general terms, the management of these types of patients is less complex than solid organ transplantation; however, some specific issues complicate it:
1.
Immunological barriers
2.
Antigen load of composite tissues
3.
Surgical stress
4.
Bleeding and fluid management
As in any other transplant discipline, VCA is performed across the major histocompatibility complex. Still, antigenicity of composite tissues and skin is considered to be much higher than any other solid organ. Shortage of compatible donors (both biological and morphological) makes tissue type matching almost impossible. HLA mismatch is common among recipients, and immunosuppression regimens need to be intense. Patients will require a multidisciplinary support to obtain the desired outcome, and significant surveillance is necessary to avoid side effects and complications.
Face transplantation is an aggressive and long surgical intervention. Duration varies, and recipient’s operative time has been reported between 15 and 30 h. During the procedure different techniques are combined (soft tissues resections, craniomaxillofacial and microsurgical techniques); the operation begins with the resection of deformed and scarred tissues, preparation of blood vessels and nerves, trimming of bony segments and osteotomies for correct skeletal alignment. It is followed by the reconstruction of the defect with a composite tissue allotransplantation (the face allograft). Revascularisation has been performed in different time sequences, although it is recommended to perform revascularisation at the early stages of VCA, in order to minimise ischaemia–reperfusion injury. The combination of different techniques in this complex reconstruction makes massive blood loss a common scenario. The surgical team should be prepared to prevent and treat appropriately important haemorrhages. Fluid management should be titrated to response, and all necessary preparations for the early postoperative period are prepared in order to avoid complications.
Still, composite tissue allotransplantation is an elective surgery procedure, which can be prepared ahead on time; recipients are normally healthy patients that do not present with organ or system dysfunctions or failure. Hence, it is considered to be a safe transplant surgery discipline that provides good outcomes provided a robust team approach is implemented. Patients are admitted to the surgical intensive care unit for postoperative management. Fluid management, enteral nutrition, immunosuppression, respiratory support, wound care and microsurgical postoperative control are necessary.
During the early postoperative period, patients may develop problems and complications caused by different conditionings, which include:
(a)
Organ and system deterioration/dysfunction
(b)
Intensive/aggressive critical-care support
(c)
Immunosuppression
Operative time has a direct impact on morbidity and complications. Long operations, massive blood transfusions, aggressive fluid replacement and respiratory infections impact systems and organ function. A critical-care holistic approach to minimise the impact of surgical stress and organ support must be implemented in order to obtain a good transition and excellent outcomes. One should not obviate that aggressive respiratory support, broad-spectrum antibiotics, immunosuppression and other interventions may produce side effects and complications. They require judicious and rigorous management and state-of-the-art nursing to prevent complications. Patients may also present with infections and acute rejection episodes, although they may be not as frequent as in other type of solid organ transplantation. In addition, acute rejection episodes do not affect the function of any vital transplanted organ (like liver, kidney, lung or heart allograft) and allow for evaluation and for a careful decision-making process (avoiding important side or toxic effects of immunosuppressive agents).
8.1 General Postoperative Support
Patients are normally admitted to the surgical intensive care unit for immediate postoperative care. In our scenario, patients are admitted to the burn intensive care unit, where they are provided with a protected environment with positive laminar flow, a robust team with physicians well versed in critical medicine support, control of microsurgical free flaps and nursing for complex plastic surgery operations (Fig. 8.1). Each team should review the positive and negative key elements for postoperative stay in different units and choose the process that best suits for a good outcome in terms of excellence in critical support, infection control and nursing (see Appendix 8.1 for specific information).
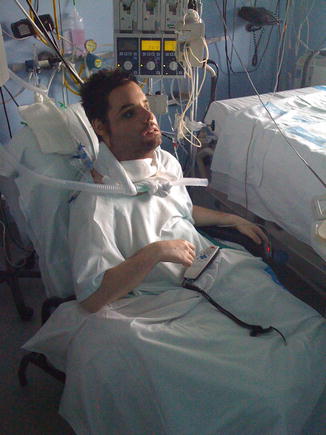

Fig. 8.1
Good postoperative nursing and critical care are key issues for correct outcomes in face VCA
Patients are admitted to the intensive care intubated and ventilated. Our clinical protocol includes an elective tracheotomy (if not already present) for correct and safe management of the airway and quick respiratory weaning. Patients are waken up and wean off the ventilator as soon as the patient is haemodynamically stable, recovers a good core temperature and does not show any sign of respiratory failure or any other organ/system dysfunction. We recommend to keep the patient sedated overnight if surgery is completed after hours, although every effort should be made to wean off the patient as soon as possible to avoid any respiratory complication.
Patients are fed through an enteral route either after weaning or with an early enteral feeding protocol if patients are not awake right after the operation. Depending on the type of deformity and the functional impact of the missing anatomy, patients will have a gastrostomy tube in place. All other patients receive enteral nutrition via an NG or NJ tube. The team should evaluate the type of transplant that is being proposed and the expected initial functional impact on swallowing and oral competence. If surgeons expect a longer postoperative time to start oral feeding, one should consider a percutaneous gastrostomy tube placement. Extreme care should be implemented in patients that are fed through a nasal tube. The transplanted nose is insensate, protective sensation may not be present until the second to third months post transplant and it will improve overtime. Therefore, patients are at risk for the development of a pressure sore on the nose (similarly to those seen in paediatric/neonatal ulcerations). Padding and protection is mandatory if the nasal route is utilised, and it should be discontinued as soon as the patient can reassume oral feeding. Invasive monitoring is maintained for as long as the patient requires haemodynamic drugs; it should be discontinued thereafter to avoid complications and infections. Central lines are nursed with extreme care to avoid line sepsis and/or phlebitis. Every effort should be made for an early removal, mandated by intravenous (IV) medication (in special systemic antibiotic prophylaxis and immunosuppression).
An aggressive rehabilitation protocol is implemented, with early passive and active range of motion, active aerobic exercise and walking. All patients should be actively encouraged to walk and exercise in order to maintain muscle tone and function. Specific rehabilitation professionals include face paralysis physiotherapists and speech pathology specialists. They start the physiotherapy protocol in the early phase, and it is continued over time for as long as the expected final functional outcome is achieved.
8.1.1 Nutritional Support
Composite tissue allotransplantation patients are healthy individuals that do not present organ failures or dysfunction before the transplant. Most of the patients are fed orally with a normal diet unless the deformity prevents so. Still, nutritional status in the pre-transplant phase should be considered normal.
Surgical stress, immunosuppression, medications and acute rejections significantly affect nutritional status and trigger a catabolic hypermetabolic response. It is necessary to maintain a well-balanced nutritional status and body weight. Acute rejections and other complications and side effects may have a relevant impact on all systems; thus, a strong physiological reserve will help to overcome these events and prolong long-term survival.
Immediately after surgery the patients are kept nil by mouth for 24 h with the exception of oral medications. In the second postoperative day, enteral nutrition via a nasojejunal tube or gastrostomy is started. Enteral nutrition is prolonged for 10 days; patients start then an oral intake for liquids and progress to a soft diet and normal diet in the following days unless dictated otherwise by the postoperative progress of patients. As soon as the patient can maintain his/her caloric requirements, enteral nutrition is discontinued completely. General principles of nutritional support are listed in Table 8.1. A re-evaluation of nutritional status after admission is mandatory; the patient’s nutritional background is determined and chronic conditions, recent weight changes and nutritional preferences noted (Table 8.2). Intolerance to enteral nutrition, including diarrhoea, may be produced by many causes, although a team member should remember that one of the most common aetiologies for intolerance and diarrhoea is infection and sepsis. VCA patients are immunosuppressed, and as such patients may not exhibit the full spectrum of signs and symptoms of impending sepsis. Infection may follow a more insidious initial phase; hence, suspicion and prompt treatment is necessary.
Table 8.1
General principles of nutritional support in VCA
All transplant patients must receive an adequate nutrition as soon as possible |
Parenteral nutrition should be used only when enteral nutrition fails |
Caloric requirements are determined by indirect calorimetry (enteral formulas may be used as an alternative) |
Limit protein intake in renal impairment |
Prevent weight loss; it is very difficult to regain body composition |
List all intake in nutritional sheet |
Adopt an early enteral feeding protocol |
Offer milk and juices |
Maintain oral nutritional supplements during hospital admission |
Do not use opioids during meals |
Perform a nutritional background evaluation on admission and control nutritional status twice a week |
Table 8.2
Initial nutritional control
Weight and height |
Lymphocyte count |
Total leukocyte count |
Haemoglobin and haematocrit |
Erythrocyte mean corpuscular volume |
Albumin, pre-albumin, magnesium, phosphate, calcium (ion), copper, zinc, protoporphyrin/haemoglobin, vitamin D, folic acid |
24 h nitrogen |
Indirect calorimetry |
8.1.2 Infection Control
There are few significant differences between VCA and solid organ transplantation (SOT) that make infection both extremely relevant and a challenging venture:
1.
VCA requires initial doses of immunosuppression significantly higher than SOT.
2.
VCA are non-sterile grafts (the skin is exposed to the environment, different amounts of mucosa are involved, sinuses and teeth are included in the graft), although the lymphoid load of the grafts is not very high.
3.
There is limited experience in infection control protocols and long-term outcomes of VCA.
In general terms, bacterial infection prophylaxis should contemplate prevention of infections in the oral and maxillofacial area. Therefore, prophylaxis includes oral bacterial flora for donors with an intensive care unit stay of more than 48 h. Incidence of viral infections, such as cytomegalovirus (CMV) and Epstein–Barr virus (EPV), should be similar to that observed in low-risk SOT (kidney transplantation). Prevention of infection against CMV and EPV and Pneumocystis jirovecii is delivered following the renal transplantation protocol (see Chap. 9).
8.1.3 Immunosuppression
The main objectives of immunosuppressive treatments include maintenance of correct graft function, absence of acute and chronic rejection, achievement of graft tolerance and the avoidance or minimisation of potential toxic or side effects of medications. Strategies for the modulation of the immune response in transplantation medicine can be divided into two main phases or stages: the induction and the maintenance immunosuppression. The whole face transplantation team must be well versed in all types of immunosuppression, timing for treatment and potential risks and side effects. Induction therapy begins in the preoperative period, and it is continued during revascularisation and immediate postoperative care.
8.1.3.1 Induction Therapy
The objective of the initial phase is to achieve an intense immunosuppression during the first week post transplant in order to reduce the probability of early acute rejection. In general terms, induction therapy is performed by the intravenous infusion of a monoclonal or polyclonal antibody, which tends to reduce the severity of any rejection episode and favours the development of long-term tolerance. During this induction period, maintenance therapy is started and monitored to adjust the doses of drugs to the serum target and the clinical response.
8.1.3.2 Maintenance Therapy
The scope of this immunosuppression protocol consists in a strategy to suppress the recipient’s immunological response against the allograft, inhibiting their ability to recognise the foreign tissues and to organise an orchestrated response. The drug regimen is adapted on an individual basis to reach the minimal doses of medications that allows for a correct immunomodulation with minimal toxicity. Immune adaptation allows for decreasing doses of medications over time while maintaining the same effect. A treatment regimen that employs multiple drugs produces a synergistic and adjunctive effect of immunosuppression minimising toxicities.
Our current immunosuppression protocol at the University Hospital Vall d’Hebron is based on a three-drug regime and induction with Thymoglobulin and prednisone.
Induction Therapy
1.
Preoperative infusion of Thymoglobulin (atg) as soon as the patient arrives to the hospital, at a dose of 2 mg/kg. Premedicate with 1 g of methylprednisolone IV, Benadryl 50 mg IV and acetaminophen 650 mg PO.
2.
Revascularisation: Administer 1 g of methylprednisolone IV, 30 min before revascularisation.