(13.1)
Where z + and z − are the charge numbers of the ions, ε r is the relative dielectric constant of the medium and T is the absolute temperature. The resulting ion pairs exhibit stable, thermodynamically distinct species. Depending on the strength of the solvent–ion interactions, ion pairs can be classified into two types: contact (tight, intimate) and solvent-separated (loose) ion pairs (Nagy and Takacs-Novak 2000).
In polar solvents with high dielectric constant such as water, ion-pair formation is also possibly obtained. Diamond (1963) demonstrated that the existing of so-called hydrophobic ion pairing (HIP). HIP is composed of two large hydrophobic ions self-assembled together by coulomb attraction, hydrophobic forces, and hydrogen bonding in polar solvents. Even though the HIP complexes display enhanced lipophilicity and thus are more suited for their potential application, it has not been paid extensive attention yet compared to the classical ion pairs mentioned above.
Hydrogen-bonded ion pairs are a special type of ion pairs. The concept of hydrogen-bonded ion pairs was brought out for better understanding the nature of the protonic acid–base interaction occurring in non-dissociating solvents (Barrow 1956). The interaction between sufficiently strong protonic acids and bases in a solvent environment, especially in solvents with low dielectric constant, tends to promote the formation of ion pairs accompanied by proton transfer of a hydrogen bond. According to the Brønsted-Lowry concept of acids and bases in aprotic solvents, two forms of simple prototropic equilibrium exist as follows (Hudson et al. 1972):
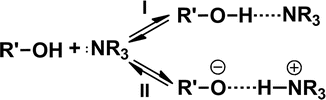

The classical electrostatic attraction was predominant in weak hydrogen bonds, so-called classical hydrogen bonds (Equilibrium I). However, on going to stronger hydrogen bonds, the contribution of the proton transfer led to the formation of ion pairs, so-called hydrogen-bonded ion pairs (Equilibrium II) (Arunan et al. 2011; Ratajczak 1972). Such a hydrogen bond is extremely polarizable. The formation of a hydrogen-bonded ion pair between a carboxylic acid and a pyridine base in benzene solution is a typical example of acid and base interaction (Barrow 1956).
13.1.2 Molecules Suitable for Ion-Pair Formation
Both drugs and counter ions have to meet certain demands in order to form ion pairs successfully. For hydrophilic ionized drugs, Neubert (1989) pointed out that the ideal counter ions needed to possess high lipophilicity, sufficient solubility in physiological compatibility, and metabolic stability, which was suitable for ion-pair formation and crossing lipid membranes in the form of ion pairs. The ion pairs formed by quaternary ammonium drugs and organic anions are typical examples of ion-pair formation (Takacs-Novak and Szasz 1999). Similarly, Miller et al. (2009) performed a study in which three lipophilic acidic counter ions were employed to give an understanding of the mechanism of ion-pair mediated membrane transport of low permeable drugs.
Besides ionized species, for stronger hydrogen bonds like OH∙∙∙N, the contribution of the proton transfer, OH · · · N ⇌ O− · · · HN+, could lead to a hydrogen-bonded ion-pair formation. Sobczyk and Paweła (1974) have demonstrated the existence of proton-transfer equilibrium under appropriate conditions by measuring the dipole moment of carboxylic acid–pyridine base complexes. The results indicated that the dipole moments of these complexes were dependent on the pK a difference (∆pK a) between carboxylic acid and pyridine base, and large dipole moment was induced by strong interaction of ion pairs. This type of interaction depends on the following factors: (a) ∆pK a of protonic acid and base, (b) specific complex solvation by solvent molecules, and (c) the influence of solvent expressed by its macroscopic dielectric permittivity. Based on the ion-pair model established by Huyskens and Zeegers-Huyskens (1964), it was predicted that a ∆pK a of 3.6–6 between protonic acid and base could lead to an almost complete shift to the proton-transfer equilibrium. A recent study (Gilli et al. 2009) also showed that ion-pair formation could be reliably predicted from ∆pK a between the donor and acceptor groups.
13.1.3 The Confirmation of Ion-Pair Formation
In theory, ion pairs are defined as binary species which exist in solution and in solid in the salt form. Such intermolecular interaction can be qualitatively inferred from the spectral characteristics. A variety of spectroscopic techniques including infrared spectroscopy (IR), nuclear magnetic resonance (NMR), ultraviolet–visible spectroscopy (UV-Vis), electron spin resonance spectroscopy (ESR), and X-ray crystallography could provide insights into ion-pair formation.
IR and NMR spectroscopy often offer experimental proofs to directly indicate the formation of ion pairs, and they are especially pronounced for hydrogen-bonded ion pairs (Barthel and Deser 1994; Biliškov et al. 2011; Habeeb 1997; Pregosin 2009). Recently, by using IR and chemical exchange two-dimensional infrared (2DIR) spectroscopy, Lee et al. (2011) investigated the contact ion pairs (CIP) assembled by Li+ and SCN−ions in N, N-dimethylformamide. In IR spectrum, the CIP formation led to a blue shift (~16 cm−1) of the CN stretch frequency of Li–SCN CIP with respect to that of free SCN− ion. Moreover, the temperature-dependent IR absorption spectra revealed that the CIP formation was an endothermic process. The CIP association and dissociation time constants (165 and 190 ps, respectively) were determined by chemical exchange 2DIR spectroscopy. The experimental results indicated that the ion-pair formation was a dynamic process in electrolyte solutions and in biological systems under physiological conditions. In the case of hydrogen-bonded ion pairs, a broad continuum, called as the Zundel continuum, is often observed in IR spectrum with extensive intermolecular hydrogen bonding, for which proton transfer is valuable. The broad band is caused by the strong hydrogen bonds in which a proton is distributed between the two hydrogen-bonded species by tunneling (Biliškov et al. 2011). According to the classical theory of hydrogen bond, a shift toward lower fields in the NMR spectrum is suggested as a criterion to confirm the formation of a hydrogen bond due to strong deshielding of the protons. Xi et al. (2012a, b) confirmed the formation of hydrogen-bonded ion pairs between weak acidic drugs and organic amines at 1:1 molar ratio by IR and 1H-NMR. In this study, teriflunomide (TEF) and lornoxicam (LOX), two weak acidic drugs with OH groups, were used as the model drugs, and various organic amines including triethylamine (TEtA), diethylamine (DEtA), N-(2′-hydroxyethanol)-piperidine (NP), diethanolamine (DEA), and triethanolamine (TEA) were employed as the counter ions, whose structure was shown in Fig. 13.1. CHCl3 and CDCl3 solutions of TEF or LOX with or without the adding of equimolar organic amines were detected, respectively, by spectroscopic methods. In IR spectra (Fig. 13.2), the absorption at ~3,400 cm−1 was assigned to stretching vibration of OH group of the two drugs. A continuum gave rise to a very broad absorption in the 3,300–2,000 cm−1 range in the presence of most of organic amines. In 1H-NMR study, compared to the signal of the proton from OH group of TEF or LOX itself (15.35 and 13.02 ppm, respectively), the proton magnetic resonance of OH in the complexes has moved toward higher field, as illustrated in Fig. 13.3. It seemed that the results were contradictory to the abovementioned classical theory of hydrogen bond in NMR. However, actually this phenomenon may be caused by strong shielding of the proton, which was a direct consequence of the intermolecular hydrogen bond interaction between drugs and organic amines instead of intramolecular hydrogen bonds in drugs. Notably, the chemical shift of OH group kept almost constant when the stoichiometric ratios of drug to organic amine were varied from 1:1 to 1:100. These results suggested that TEF and LOX have been integrated sufficiently into ion pairs at the equimolar ratio.
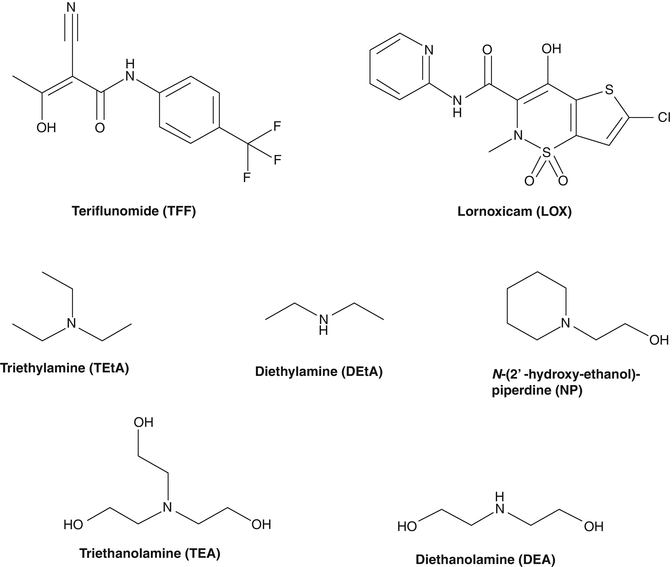
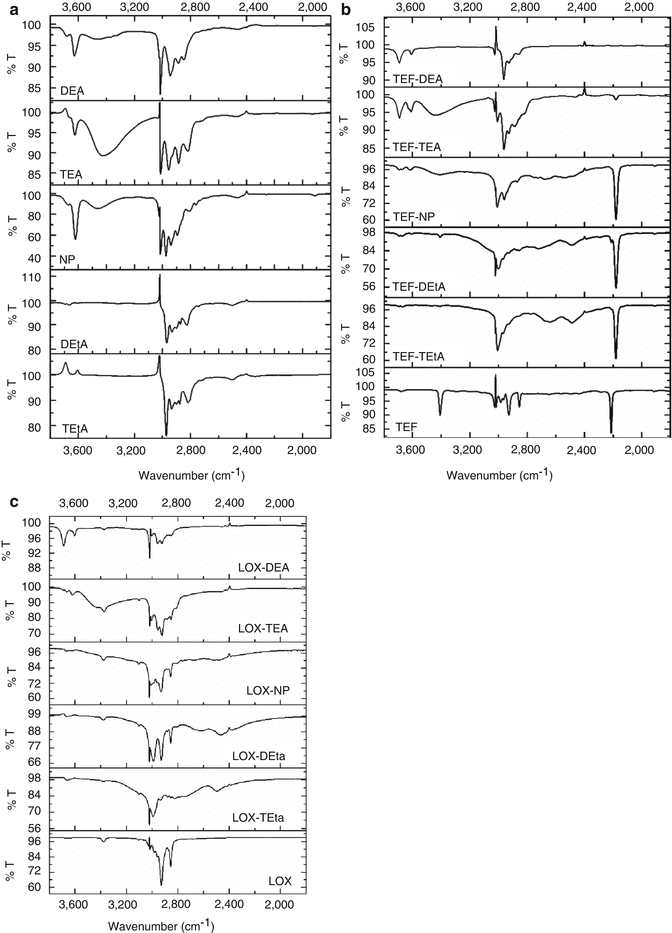
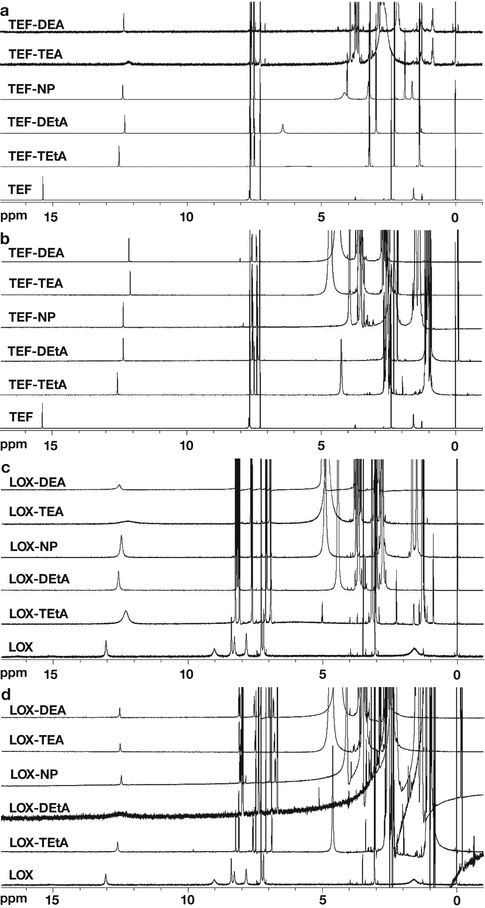

Fig. 13.1
The chemical structure of teriflunomide (TEF), lornoxicam (LOX), triethylamine (TEtA), diethylamine (DEtA), N-(2′-hydroxyethanol)-piperidine (NP), diethanolamine (DEA), and triethanolamine (TEA)

Fig. 13.2
Infrared spectra of organic amines, TEF and LOX in CHCl3: (a) organic amines which was equimolar with test drug; (b) TEF with or without equimolar organic amines; (c) LOX with or without equimolar organic amines

Fig. 13.3
1H-NMR spectra of TEF and LOX with or without organic amines in CDCl3 at different molar ratios: (a) TEF, amines = 1:1; (b) TEF, amines = 1:100; (c) LOX, amines = 1:1; (d) LOX, amines = 1:100
In addition, UV-Vis, ESR spectra in solution, and X-ray crystallography also have been employed for measuring the electronic changes in ion-pair formation process (Lü et al. 2005; Pal et al. 2010). Hudson et al. (1972) found that when weak acidic 3,4-dinitrophenol encountered organic amines at different molar ratio, a series of characteristic bathochromic shifts in UV absorption spectra were presented in going from free acid to a hydrogen-bonded complex, to an ion pair, to a solvated ion pair or a solvated anion. Lü et al. (2005) determined the crystal structures of pure ion-pair salts K(LC)+//DNB− and K(LE)+DNB− by X-ray crystallography. In the near-IR spectral analysis, they found that there were the same patterns of vibronic progressions for distinguishing the “separated” from the “contact” ion pair in both of the crystalline solid state and THF solution state, which ensured that the same X-ray structures persist in solution. Most importantly, in this study, the labilities of these dynamic ion pairs in solution were thoroughly elucidated by the temperature-dependent ESR spectral changes. Compared with other methods, X-ray crystallography can provide definitive structural information via analyzing the diffraction pattern of single crystal ion-pair salts. In another study, Fang et al. (2004) prepared the crystals of ion-pair complexes with an equimolar ratio of mefenamic acid (MH) and alkanolamines by removing the solvent in vacuo and subsequently confirmed that these complexes were associated with hydrogen bonds using X-ray crystallography (Fig. 13.4).
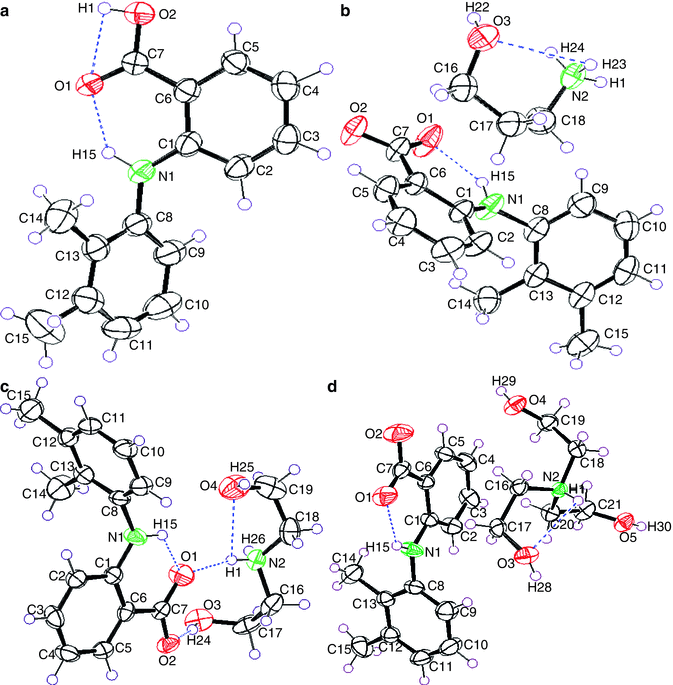

Fig. 13.4
Stereoscopic view of (a) MH, (b) MH- propanolamine, (c) MH-DEA, and (d) MH-TEA, showing atom numbering, 50 % thermal probability ellipsoids, and hydrogen bonds. Dashed lines indicate the intermolecular hydrogen bonding
13.1.4 The Effect of Ion-Pair Formation on Skin Permeation
13.1.4.1 The Mechanism of Skin Penetration Enhancement by Ion-Pair Formation
The effect of ion-pair formation on skin permeation is complex, and its mechanism has not been thoroughly clarified. Generally, the skin penetration enhancement by ion-pair approach with suitable counter ions is mainly dependent on the physicochemical properties of the counter ions (e.g., lipophilicity, pK a, and structure) and the solubility of ion pairs in donor medium.
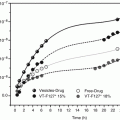
The extent of penetration enhancement by ion-pair formation is strongly related to the lipophilicity of the ion pairs and the properties that depend on the lipophilicity of the selected counter ions. A series of studies performed by Neubert et al. (Neubert et al. 1984; Neubert and Dittrich 1989; Neubert and Fischer 1991) have made great contribution to the understanding of how hydrophilic ionized drugs penetrate across lipid membranes together with lipophilic counter ions. These studies showed that the partition coefficient of the hydrophilic drugs, buformine, quinine, pholedrine, and bretylium, was markedly increased by more than twofold after the formation of ion pairs with lipophilic ions, and thereby the transport of ionized drugs across an artificial lipid membrane (dodecanol collodion membrane) could be enhanced. Moreover, it was found that the counter ions could be accumulated in the lipid membrane due to their high lipid solubility and that they acted as carriers for the ionized drugs. Besides the increased transport of ionized drugs, the counter transport of protons and lithium ions, respectively, was also observed. Nam et al. (2011) also provided a similar result in the skin permeation of hydrophilic and highly ionized risedronate (RIS) with three lipophilic basic counter ions, l-arginine, l-lysine, and diethylenetriamine, at different molar ratios. To varying degree, all the counter ions could enhance the solubility of RIS in xylene, a lipophilic solvent. Although RIS ion pairs are slightly unstable in the aqueous solution, they had a remarkable enhancing effect on RIS penetration from the aqueous solution into hairless mouse skin, and RIS-diethylenetriamine ion pair brought out the largest enhancement ratio (ER), up to 36-fold compared to only RIS.
As to lipophilic drugs possessing some polar functional groups, e.g., –COOH, −OH, and –NH2, their skin permeation can also be enhanced by hydrogen-bonded ion-pair formation (Cheong and Choi 2002; Green et al. 1989; Kamal et al. 2007; Nogueira et al. 2011). However, for those lipophilic drugs, their lipophilicity can be decreased by ion-pair formation with small molecular weight relative hydrophilic counter ions (Fang et al. 2003). The effect of the organic amines including monoethanolamine (MEA), DEA, TEA, and propanolamine (PPA) on the penetration of mefenamic acid (MH) across hairless rat skin from the lipophilic mixed solvent of isopropyl myristate (IPM) and ethanol (9:1). The n-octanol/water partition coefficients (log K o/w) at 32 °C of MH and its corresponding ion pairs, MH-MEA, MH-DEA, MH-TEA, and MH-PPA, were 3.31, 0.79, 0.74, 1.99, and 0.66, respectively, which indicated that these complexes were relatively hydrophilic compared with MH. Hence, the transdermal delivery of MH was significantly enhanced by the formation of hydrogen-bonded ion pairs, and the ER values of these ion pairs were 279, 48, 84, and 357, respectively. Obviously, the reduced lipophilicity of the complexes has facilitated the partition from the SC to the ED and consequently enhanced drug delivery through the skin. These results suggested that a major part of ion pairs remained the integrity of ion pair during the process of crossing the lipophilic SC and the hydrophilic ED until they reach the receptor compartment. The subsequent studies done by Fang’s group further confirmed this point of view (Table 13.1).
Table 13.1
The enhancement ratio (ER) of ion pairs of drugs with different amines
Drugs | Counter ions | ERa |
|---|---|---|
Teriflunomide in isopropyl palmitateb | None | 1.00 |
Diethylamine | 2.47 | |
Triethylamine | 12.69 | |
Triethanolamine | 1.44 | |
Diethanolamine | 1.15 | |
N-(2′-hydroxyethanol)-piperidine | 4.54 | |
Lornoxicam in isopropyl palmitatec | None | 1.00 |
Diethylamine | 13.63 | |
Triethylamine | 19.52 | |
Triethanolamine | 4.92 | |
Diethanolamine | 13.77 | |
N-(2′-hydroxyethanol)-piperidine | 12.08 | |
Flurbiprofen in 10 % EtOH/isopropyl myristated | None | 1.00 |
Diethylamine
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|