Many signs of cutaneous photodamage are amenable to treatment with a variety of ablative and non-ablative lasers, light sources, and fractional photothermolysis.
Ablative laser skin resurfacing offers the most substantial clinical improvement, but is associated with several weeks of postoperative recovery.
Severe side effects and complications after ablative laser skin resurfacing can be minimized by careful patient selection, proper surgical technique, and meticulous postoperative care.
Non-ablative laser skin remodeling is a good alternative for patients who desire modest improvement of photodamaged skin without significant post-treatment recovery.
The noninvasive nature of fractional photothermolysis treatment, coupled with an excellent side effect profile, makes it an attractive alternative to ablative laser techniques.
Good candidates for non-ablative laser and light-source treatments are patients with cutaneous photodamage and realistic clinical expectations.
With ongoing advancements in laser technology and techniques, improved clinical outcomes with minimal postoperative recovery will be realized.
Introduction
A wide variety of lasers and light-based sources is available to treat cutaneous photodamage including ablative and non-ablative lasers, light sources, and fractional photothermolysis. |
Years of damaging ultraviolet (UV) light exposure manifests clinically as a sallow complexion with roughened surface texture and variable degrees of dyspigmentation, telangiectasias, wrinkling, and skin laxity.1,2 Histologically, these extrinsic aging effects are usually limited to the epidermis and upper papillary dermis and are therefore amenable to treatment with a variety of ablative and non-ablative lasers and light-sources.3
History of Procedures
Selective photothermolysis theory of laser-tissue interaction is used to create thermal destruction of target tissue without unwanted conduction of heat to surrounding structures by selecting the appropriate laser wavelength and pulse duration. The first system specifically developed for cutaneous laser resurfacing was the pulsed carbon dioxide (CO2) laser. The short-pulsed erbium:yttrium–aluminum–garnet (Er:YAG) laser was subsequently used as an alternative to the CO2 laser to minimize the recovery period and limit side effects while maintaining clinical benefit. |
Although dermatologic laser surgery is nearly five decades old, the field was revolutionized in 1983 when Anderson and Parrish elucidated the principles of selective photothermolysis.4 This basic theory of laser-tissue interaction explains how selective tissue destruction is possible. In order to effect precise thermal destruction of target tissue without unwanted conduction of heat to surrounding structures, the proper laser wavelength must be selected for preferential absorption by the intended tissue chromophore. Furthermore, the pulse duration of laser emission must be shorter than the thermal relaxation time of the target–thermal relaxation time (TR) being defined as the amount of time necessary for the targeted structure to cool to one-half of its peak temperature immediately after laser irradiation. The delivered fluence (energy density) must also be sufficiently high to cause the desired degree of thermal injury to the skin. Thus, the laser wavelength, pulse duration, and fluence each must be carefully chosen to achieve maximal target ablation while minimizing surrounding tissue damage.
The first system specifically developed for cutaneous laser resurfacing was the pulsed carbon dioxide (CO2) laser, which was approved by the Food and Drug Administration (FDA) in 1996. Earlier CO2 systems were continuous-wave (CW) lasers which were effective for gross lesional destruction,5,6 but were unable to reliably ablate fine layers of tissue because of excessive tissue heating which produced unacceptably high rates of scarring and pigmentary alteration.7–9 The unpredictable nature of the CW lasers prevented their widespread use in facial resurfacing procedures. With the subsequent development of high-energy, pulsed lasers it became possible to safely apply higher energy densities with exposure times that were shorter than the thermal relaxation time of water-containing tissue, thus lowering the risk of thermal injury to surrounding non-targeted structures.3,10
The short-pulsed erbium:yttrium–aluminum–garnet (Er:YAG) laser was subsequently FDA-approved for cutaneous resurfacing as an alternative to the CO2 laser in an attempt to minimize the recovery period and limit side effects while maintaining clinical benefit.
In response to growing public interest in minimally-invasive treatment modalities, non-ablative laser and light source technology was later developed. Rapid advances in non-ablative technology have produced several lasers and light-based sources capable of improving fine facial rhytides, dyspigmentation, and telangiectasia associated with cutaneous photodamage.
The armamentarium of lasers and light-based sources currently available to treat cutaneous photodamage is larger than ever before (Table 1). The most appropriate technique depends upon the severity of photodamage and rhytides, the expertise of the laser surgeon, and the expectations and lifestyle of the individual patient.
Table 1
Lasers and light sources for skin resurfacing
Laser type | Wavelength (nm) | |
|---|---|---|
Ablative | CO2 (pulsed) Er:YAG (pulsed) | 10,600 2,940 |
Non-ablative | Pulsed dye Nd:YAG, Q-switched Nd:YAG, long-pulsed Diode, long-pulsed Er:glass, long-pulsed Intense pulsed light source | 585–595 1,064 1,320 1,450 1,540 515–1,200 |
Fractional | Er-doped fiber | 1,550 |
Ablative Laser Skin Resurfacing
Indications and Contraindications
Indications Mild-to-moderate rhytides, preferably in non-movement-associated areas. Other signs of photodamage (e.g., dyspigmentation and keratoses). Shallow atrophic scars. Superficial skin lesions. Contraindications Patients with unrealistic expectations. Patients with perpetual sun exposure. Active bacterial, viral, fungal infection or inflammatory skin conditions involving the skin areas to be treated. Patients with prior lower blepharoplasties using an external approach are at greater risk of ectropion formation after infraorbital ablative laser treatment. Patients with darker skin tones (skin phototype IV–VI) have a high incidence of postoperative hyperpigmentation. Concomitant isotretinoin use could potentially lead to an increased risk of hypertrophic scarring. Patients with a propensity to scar will be at greater risk for postoperative scarring. |
The ideal patient for ablative laser skin resurfacing has a fair complexion (skin phototype I or II), exhibits cutaneous lesions that are amenable to treatment, and has realistic expectations of the resurfacing procedure. Adequate preoperative patient evaluation and education are absolute essentials to avoid pitfalls and optimize the clinical outcome (Table 2). Proper patient selection is paramount as ablative laser resurfacing can be complicated by a prolonged postoperative recovery, pigmentary alteration, or unexpected scarring. The patient’s emotional ability to tolerate an extended convalescence is an important factor in determining the most appropriate choice of laser. Although CO2 and modulated Er:YAG lasers often produce the most dramatic clinical results, some patients may be unable to tolerate the intensive recovery period. For patients unable or unwilling to withstand extended postoperative healing, a short-pulsed Er:YAG laser or application of a non-ablative or fractional laser procedure may be a more suitable choice.
Table 2
Ablative laser resurfacing: patient selection, risks, and precautions
Preoperative patient evaluation |
|---|
Are the lesions amenable to ablative laser skin resurfacing? All suspicious lesions require biopsy before treatment |
Has the patient ever had the areas treated before? Ablative laser resurfacing can unmask hypopigmentation or fibrosis produced by prior dermabrasion, cryosurgery, or phenol peels. Patients with prior lower blepharoplasties using an external approach are at greater risk of ectropion formation after infraorbital ablative laser treatment |
What is the patient’s skin phototype? Patients with paler skin tones (skin phototype I or II) have a lower incidence of postoperative hyperpigmentation than do patients with darker skin tones |
Does the patient have a history of herpes labialis? All patients should be treated with prophylactic antiviral medication before perioral treatment, because reactivation and/or dissemination of prior herpes simplex infection can occur. The de-epithelialized skin is also particularly susceptible to primary inoculation by herpes simplex virus |
Does the patient have an autoimmune disease or other immunologic deficiency? Intact immunologic function and collagen repair mechanisms are necessary to optimize the tissue-healing response due to the prolonged recovery associated with ablative resurfacing |
Is the patient taking any medications that are contraindicated? Concomitant isotretinoin use could potentially lead to an increased risk of postoperative hypertrophic scar formation due to its detrimental effect on wound healing and collagenesis. A safe interval between the use of oral retinoids and ablative laser skin resurfacing is difficult to determine; however, most advocate a delay in treatment for at least 6 months after discontinuation of the drug |
Does the patient have a tendency to form hypertrophic scars or keloids? Patients with a propensity to scar will be at greater risk of scar formation after treatment, independent of the laser’s selectivity and the operator’s expertise |
Does the patient have realistic expectations of the procedure and adhere to postoperative instructions? Patients who cannot physically or emotionally handle the prolonged postoperative course should be dissuaded from pursuing ablative laser skin treatment |
Techniques
Preoperative Management
Adequate preoperative patient evaluation and education. Oral antibiotic prophylaxis as indicated. |
There is no consensus among laser experts regarding the most appropriate preoperative regimen for ablative laser skin resurfacing. The use of topical retinoic acid compounds, hydroquinone bleaching agents, or α-hydroxy acids for several weeks before laser treatment has been touted as a means of speeding recovery and decreasing the incidence of postinflammatory hyperpigmentation.11 Topical tretinoin enhances penetration of chemicals through the skin and has been shown to accelerate postoperative re-epithelialization after dermabrasion or deep chemical peels.12 However, because ablative laser-induced wounds are intrinsically different from those created by physically destructive methods, laser skin penetration is not typically affected by the topical application of any of these medications. In addition, being that postinflammatory hyperpigmentation is relatively common after ablative cutaneous laser resurfacing, many laser surgeons originally believed that the prophylactic use of topical bleaching agents would reduce the incidence of this side effect, but investigators subsequently demonstrated that the preoperative use of topical tretinoin, hydroquinone, or glycolic acid had no effect on the incidence of postablative laser hyperpigmentation.13
Due to the moist, de-epithelialized state of ablative laser-resurfaced skin and the possibility of bacterial contamination and overgrowth, many laser surgeons advocate oral antibiotic prophylaxis; however, this practice remains controversial due to the results of a controlled study that demonstrated no significant change in post-laser resurfacing infection rate in patients treated with prophylactic antibiotics.14 The most common infectious complication is a reactivation of labial herpes simplex virus (HSV), most likely caused by the thermal tissue injury and epidermal disruption produced by the laser.15,16 Any patient undergoing full-face or perioral ablative resurfacing should receive antiviral prophylaxis even when a history of HSV is denied. It is impossible to predict who will develop HSV reactivation, because a negative cold sore history is an unreliable method to determine risk and many patients do not remember having had an outbreak or are asymptomatic HSV carriers. Oral antiviral agents, such as acyclovir, famciclovir, and valacyclovir are effective agents against HSV infection, although severe (disseminated) cases may require intravenous therapy. Patients should begin prophylaxis by the day of surgery and continue for 7–10 days postoperatively.
Description of the Technique
Carbon Dioxide (CO2) Laser
Areas with thinner skin (e.g., periorbital) require fewer laser passes. Non-facial (e.g., neck, chest) areas should be avoided due to paucity of pilosebaceous units with diminished capacity for re-epithelialization. Avoidance of pulse stacking in order to decrease risk of scarring. |
The Ultrapulse (Lumenis Corp, Yokeam, Israel), one of the first high-energy, pulsed CO2 laser systems developed, emits individual high energy pulses (peak energy densities of 500 mJ in 600 μs–1 ms). Its earliest competitor, the SilkTouch (Lumenis Corp, Yokeam, Israel), was a continuous-wave CO2 system with a microprocessor scanner that continuously moved the laser beam so that light did not dwell on any one area for more than 1 ms. The peak fluences delivered per pulse or scan ranged from 4 to 5 J/cm2, which were the energy densities determined to be necessary for complete tissue vaporization.7,17–19 Studies with these and other pulsed or scanned CO2 laser systems showed that after a typical skin resurfacing procedure, water-containing tissue was vaporized to a depth of approximately 20–60 μm, producing a zone of thermal damage ranging 20–150 μm.7,18,20–22
The depth of ablation correlates directly with the number of passes performed and usually is confined to the epidermis and upper papillary dermis; however, stacking of laser pulses by treating an area with multiple passes in rapid succession or by using a high overlap setting on a scanning device can lead to excessive thermal injury with subsequent increased risk of scarring.15,23,24 An ablative plateau is reached with less effective tissue ablation and accumulation of thermal injury. This effect is most likely caused by reduced tissue water content after initial desiccation, resulting in less selective absorption of energy.24 The avoidance of pulse stacking and incomplete removal of partially desiccated tissue is paramount to prevention of excessive thermal accumulation with any laser system.
The objective of ablative laser skin resurfacing is to vaporize tissue to the papillary dermis. Limiting the depth of penetration decreases the risk for scarring and permanent pigmentary alteration. When choosing treatment parameters, the surgeon must consider factors such as the anatomic location to be resurfaced, the skin phototype of the patient, and previous treatments delivered to the area.17,25 In general, areas with thinner skin (e.g., periorbital) require fewer laser passes and non-facial (e.g., neck, chest) laser resurfacing should be avoided due to the relative paucity of pilosebaceous units in these areas.25 To reduce the risk of excessive thermal injury, partially desiccated tissue should be removed manually with wet gauze after each laser pass to expose the underlying dermis.24
The clinical and histologic benefits of cutaneous laser resurfacing are numerous. With the CO2 laser, most studies have shown at least a 50% improvement over baseline in overall skin tone and wrinkle severity (Fig. 1a, b).10,26–30 The biggest advantages associated with CO2 laser skin resurfacing are the excellent tissue contraction, hemostasis, prolonged neocollagenesis and collagen remodeling that it provides. Histologic examination of laser-treated skin demonstrates replacement of epidermal cellular atypia and dyplasia with normal, healthy epidermal cells from adjacent follicular adnexal structures.7,21 The most profound effects occur in the papillary dermis, where coagulation of disorganized masses of actinically-induced elastotic material are replaced with normal compact collagen bundles arranged in parallel to the skin’s surface.31,32 Immediately after CO2 laser treatment, a normal inflammatory response is initiated, with granulation tissue formation, neovascularization, and increased production of macrophages and fibroblasts.21
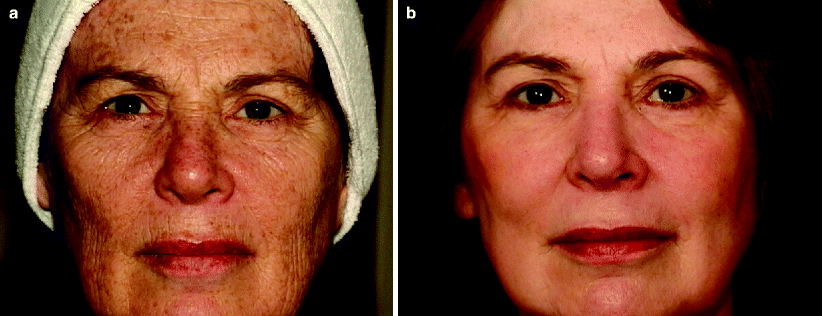

Fig. 1
CO2 laser resurfacing (a) before and (b) after
Persistent collagen shrinkage and dermal remodeling are responsible for much of the continued clinical benefits observed after CO2 resurfacing and are influenced by several factors.33,34 Thermal effects of laser irradiation of skin produce collagen fiber contraction at temperatures ranging from 55°C to 62°C through disruption of interpeptide bonds resulting in a conformational change to the collagen’s basic triple helical structure.35,36 The collagen molecule is thereby shortened to approximately one third of its normal length. The laser-induced shrinkage of collagen fibers may act as the contracted scaffold for neocollagenesis, leading to subsequent production of the newly shortened form. In turn, fibroblasts that migrate into laser wounds after resurfacing may up-regulate the expression of immune modulating factors that serve to enhance continued collagen shrinkage.37
The CO2 resurfacing laser is a most effective tool for improving photo-induced facial rhytides; however, dynamic rhytides are not as amenable to laser treatment. Many patients experience recurrence of movement-associated rhytides (particularly in the glabellar region) within 6–12 months postoperatively. Thus, cosmetic denervation with intramuscular injections of botulinum toxin type A is often used concomitantly with laser resurfacing to provide prolonged clinical improvement.38
Absolute contraindications to CO2 laser skin resurfacing include active bacterial, viral, or fungal infection or an inflammatory skin condition involving the skin areas to be treated. Isotretinoin use within the preceding 6–month period or history of keloids also are considered contraindications to CO2 laser treatment because of the unpredictable tissue healing response and greater risk for scarring.39,40
In an attempt to address many of the difficulties associated with the use of multiple-pass CO2 laser skin resurfacing, refinements in surgical technique were subsequently developed. Single-pass CO2 laser treatment was shown to effect faster re-epithelialization and an improved side effect profile.41 Rather than remove partially desiccated tissue (as was typical with multiple-pass procedures), the lased skin was left intact to serve as a biologic wound dressing. Additional laser passes could then be applied focally in areas with more severe photodamage in order to limit unnecessary thermal and mechanical trauma to uninvolved skin. Subsequent reports have substantiated the improved side effect profile of this less aggressive procedure.42–44
Erbium:Yttrium–Aluminum–Garnet (Er:YAG) Laser
Typical fluences range from 5 to 15 J/cm2, depending on the degree of photodamage and anatomic location. When lower fluences are applied, it is often necessary to perform multiple passes to ablate the entire epidermis. Shorter pulse durations are used for tissue ablation and longer pulses are used to effect coagulation and expand zones of thermal injury. |
The Er:YAG laser is a more ablative tool that emits light at 2,940 nm, corresponding well to the 3,000 nm absorption peak of water. The absorption coefficient of the Er:YAG is 12,800 cm−1 (compared with 800 cm−1 for the CO2 laser), making it 12–18 times more efficiently absorbed by water-containing tissue than is the CO2 laser.45 The pulse duration (averaging 250 μs) is also much shorter than that of the CO2 laser, resulting in decreased thermal diffusion, less effective hemostasis, and increased intraoperative bleeding which can hamper deeper dermal treatment. Because of limited thermal skin injury, the amount of collagen contraction is also reduced with Er:YAG treatment (1–4%) compared to that observed with CO2 laser irradiation.11,46
The erbium’s efficient rate of absorption, short exposure duration, and direct relationship between fluence delivered and amount of tissue ablated leads to 2–4 μm of tissue vaporization per Joule per square centimeter, producing a shallow level of tissue ablation. Much narrower zones of thermal necrosis, averaging only 20–50 μm, are therefore produced.45,47–49 Laser-induced ejection of desiccated tissue from the target site typically produces a distinctive popping sound. Thermal energy is confined to the selected tissue, with minimal collateral thermal damage. Because little tissue necrosis is produced with each pass of the laser, manual removal of desiccated tissue is often unnecessary.
The short-pulsed erbium laser fluences used most often range from 5 to 15 J/cm2, depending on the degree of photodamage and anatomic location. When lower fluences are used, it is often necessary to perform multiple passes to ablate the entire epidermis. The ablation depth with the short-pulsed Er:YAG does not diminish with successive passes, because the amount of thermal necrosis is minimal with each pass. It takes three to four times as many passes with the short-pulsed Er:YAG laser to achieve similar depths of penetration as with one pass of the CO2 laser at typical treatment parameters.3,11 To ablate the entire epidermis with the short-pulsed Er:YAG laser at 5 J/cm2, at least two or three passes must be used which increases the possibility of uneven tissue penetration. Deeper dermal lesions or areas of the face with extreme photodamage and extensive dermal elastosis may require up to nine or ten passes of the short-pulsed Er:YAG laser, whereas the CO2 laser would effect similar levels of tissue ablation in two or three passes.7,18,45
Pinpoint bleeding caused by inadequate hemostasis and tissue color change with multiple Er:YAG passes can impede adequate clinical assessment of wound depth. Irradiated areas whiten immediately after treatment and then quickly fade. These factors renders far more difficult for the surgeon to determine treatment endpoints and thus requires extensive knowledge of laser–tissue interaction.
Conditions amenable to short-pulsed Er:YAG laser resurfacing include superficial epidermal or dermal lesions, mild photodamage and subtle dyspigmentation. The major advantage of short-pulsed Er:YAG laser treatment is its shorter recovery period. Re-epithelialization is completed within an average of 5.5 days, compared with 8.5 days for multiple-pass CO2 procedures.18,47 Postoperative pain and duration of erythema are reduced after short-pulsed Er:YAG laser resurfacing, with postoperative erythema resolving within 3–4 weeks. Because there is less thermal injury and trauma to the skin, the risk of pigmentary disturbance is also decreased, making the short-pulsed Er:YAG laser a good alternative in patients with darker skin phototypes.3,50 The major disadvantages of the short-pulsed Er:YAG laser are its limited ability to effect significant collagen shrinkage and its failure to induce new and continued collagen formation postoperatively.3,47,51 The final clinical result is typically less impressive than that produced by CO2 laser skin resurfacing for deeper rhytides. However, for mild photodamage, improvement of approximately 50% is typical (Fig. 2a, b). Although clinical and histologic effects are less impressive than those produced with the CO2 laser, short-pulsed Er:YAG laser skin resurfacing still affords modest improvement of photodamaged skin with a shorter recovery time.17,47
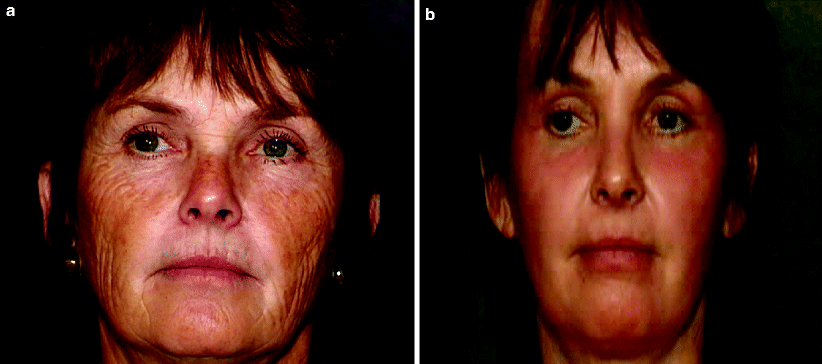

Fig. 2
Erbium:YAG laser resurfacing (a) before and (b) after
To address the limitations of the short-pulsed Er:YAG laser, modulated Er:YAG lasers systems were developed to improve hemostasis and increase the amount of collagen shrinkage and remodeling effected. The Er:YAG–CO2 hybrid laser system delivers both ablative Er:YAG and coagulative CO2 laser pulses. The Er:YAG component generates fluences up to 28 J/cm2 with a 350 μs pulse duration, while excellent hemostasis is provided by the CO2 component which can be programmed to deliver 1–100 ms pulses at 1–10 W power. Zones of thermal necrosis measuring as much as 50 μm have been observed depending on the treatment parameters used and significant increase in collagen thickness has been noted 3 months after four passes with this hybrid technology.52 Another modulated Er:YAG device is a dual-mode Er:YAG laser that emits a combination of short (200–300 μs) pulses and long coagulative pulses to achieve tissue ablation depths of up to 200 μm per pass. The output from the two Er:YAG laser heads are combined into a single stream in a process called optical multiplexing.53 The desired depth of ablation and coagulation can be programmed by the laser surgeon into the touch-screen control panel. Several investigators have studied the histologic effects of dual-mode Er:YAG laser resurfacing and found a close correlation between the programmed and actual measured depths of ablation.54,55 The actual zones of thermal injury correlate well to the first pass with decreasing coagulative efficiency on subsequent passes. The variable-pulsed Er:YAG laser system delivers pulse durations ranging 500 μs–10 ms. Shorter pulse durations are used for tissue ablation and longer pulses are used to effect coagulation and zones of thermal injury similar to the CO2 laser.53,56
Since the modulated Er:YAG lasers were developed to produce a greater thermal effect and tissue contraction than their short-pulsed predecessors, investigators compared collagen tightening induced by the CO2 laser with that of the CO2–Er:YAG hybrid laser system.57 Intraoperative contraction of approximately 43% was produced after three passes of the CO2 laser, compared with 12% contraction following Er:YAG irradiation. At 4 weeks; however, the CO2 and Er:YAG laser treated sites were contracted to the same degree, highlighting the different mechanisms of tissue tightening observed after laser treatment. Immediate thermal-induced collagen tightening was the predominant response seen after CO2 irradiation, whereas modulated Er:YAG laser resurfacing did not produce immediate intraoperative contraction but instead induced slow collagen tightening.53,57
Postoperative Management
During the re-epithelialization process, an open- or closed-wound technique can be used. Ice pack application, anti-inflammatory and pain medications should be prescribed. Early recognition and treatment of side effects (e.g., topical bleaching creams for hyperpigmentation). |
Wound care during the immediate postoperative period is vital to the successful recovery of ablative laser-resurfaced skin. During the re-epithelialization process, an open- or closed-wound technique can be prescribed. Partial-thickness cutaneous wounds heal more efficiently and with a reduced risk of scarring when maintained in a moist environment because the presence of a dry crust or scab impedes keratinocyte migration.58 Although there is consensus on this principle, disagreement exists regarding the optimal dressing for the laser-ablated wound. The “open” technique involves frequent application of a thick healing ointment to the de-epithelialized skin surface; whereas occlusive or semi-occlusive dressings are placed directly on the lased skin in the “closed” technique. While the open technique facilitates easy wound visualization, the closed technique requires less patient involvement and may also decrease postoperative pain. Proposed advantages of a closed wound care regimen include increased patient comfort, decreased erythema and edema, increased rate of re-epithelialization, and decreased patient involvement in wound management.58,59 On the other hand, additional expense and a higher risk of infection have been associated with the use of these dressings.3,60,61
In addition to postoperative wound care, ice pack application and anti-inflammatory medications should be prescribed during this time. Furthermore, pain medication is particularly important for ablative laser-resurfaced patients during the first few postoperative days.
Adverse Events
Side Effects/Complications Mild: Prolonged erythema, milia, acne, contact dermatitis. Moderate: Infection (bacterial, viral, fungal), hyperpigmentation. Severe: Hypopigmentation, hypertrophic scarring, ectropion. Prevention and Treatment Adequate preoperative patient evaluation and education are absolute essentials to avoid pitfalls and optimize clinical outcomes. Preoperative examination to determine eyelid skin laxity/elasticity to prevent ectropion. Avoidance of pulse stacking, scan overlapping, and incomplete removal of partially desiccated tissue to prevent hypertrophic scarring. Topical agents such as retinoic acid derivatives, glycolic acid, salicylic acid, fragrance-containing or chemical-containing cosmetics and sunscreens should be strictly avoided in the early postoperative period until substantial healing has occurred. Oral antibiotics may be prescribed for acne flares that do not respond to topical preparations. Any patient undergoing full-face or perioral ablative resurfacing should receive antiviral prophylaxis, even if a history of HSV is denied. Postinflammatory hyperpigmentation can be hastened with the postoperative use of a variety of topical agents, including hydroquinone, retinoic, azeleic, and glycolic acid. Regular sunscreen use is important during the healing process to prevent further skin darkening. |
Side effects associated with ablative laser skin resurfacing vary and are related to the expertise of the laser surgeon, the body area treated, and the skin phototype of the patient (Table 3). Certain tissue reactions, such as erythema and edema, are expected in the immediate postoperative period and are not considered adverse events. Erythema can be intense and may persist for several months after the procedure. The degree of erythema correlates directly with the depth of ablation and the number of laser passes performed.3,16 It may also be aggravated by underlying rosacea or dermatitis. Postoperative erythema resolves spontaneously but can be reduced with the application of topical ascorbic acid which may serve to decrease the degree of inflammation.62,63 Its use should be reserved for at least 4 weeks after the procedure in order to avoid irritation. Similarly, other topical agents such as retinoic acid derivatives, glycolic acid, fragrance-containing or chemical-containing cosmetics and sunscreens should be strictly avoided in the early postoperative period until substantial healing has occurred.16 Adequate preoperative patient evaluation and education are absolute essentials to avoid pitfalls and to optimize clinical outcomes.
Table 3
Side effects and complications of ablative laser skin resurfacing
Expected side effects | Complications | ||
|---|---|---|---|
Mild | Moderate | Severe | |
Erythema | Prolonged erythema | Infection (bacterial, viral, fungal) | Permanent hypopigmentation |
Edema | Milia | Transient hyperpigmentation | Hypertrophic scarring |
Pruritus | Acne Dermatitis | Ectropion | |
Mild side effects of laser resurfacing include milia formation and acne exacerbation which may be caused by the use of occlusive dressings and ointments during the postoperative period, particularly in patients who are prone to acne.15,16,25,60 Milia and acne usually resolve spontaneously as healing progresses and the application of thick emollient creams and occlusive dressings ceases. Oral antibiotics may be prescribed for acne flares that do not respond to topical preparations.16,30,60 Contact allergies, irritant or allergic, can also develop from various topical medications, soaps, and moisturizers used postoperatively. Most of these reactions are irritant in nature due to decreased barrier function of the newly resurfaced skin.16,64
Wound infections associated with ablative laser resurfacing include Staphylococcus, Pseudomonas, or cutaneous candidiasis and should be treated aggressively with an appropriate systemic antibiotic or antifungal agent.61 The use of prophylactic antibiotics remains controversial.14 After CO2 resurfacing, approximately 7% of patients develop a localized or disseminated form of HSV.16 These infections develop within the first postoperative week and can present as erosions without intact vesicles because of the denuded condition of newly lased skin. Even with appropriate prophylaxis, a herpetic outbreak still can occur in up to 10% of patients and must be treated aggressively.17 In order to prevent this complication, patients should begin prophylaxis by the day of surgery and continue for 7–10 days postoperatively.
The most severe complications associated with ablative cutaneous laser resurfacing include hypertrophic scar and ectropion formation.15,16 Although the risk of scarring has been significantly reduced with pulsed laser technology (compared to the continuous wave systems), inadvertent pulse stacking or scan overlapping, as well as incomplete removal of desiccated tissue between laser passes can cause excessive thermal injury that could increase the development of fibrosis. Focal areas of bright erythema, with pruritus, particularly along the mandible, may signal impending scar formation.25,65 Ultrapotent (class I) topical corticosteroid preparations should be applied to decrease the inflammatory response. A pulsed dye laser also can be used to improve the appearance and symptoms of laser-induced burn scars.65
Ectropion of the lower eyelid after periorbital laser skin resurfacing is rarely seen, but if encountered, usually requires surgical correction.25 It is more likely to occur in patients who have had previous lower blepharoplasty or other surgical manipulation of the periorbital region. Preoperative examination is essential to determine eyelid laxity and skin elasticity. If the infraorbital skin does not return briskly to its normal resting position after a manual downward pull (snap test), then ablative laser treatment near the lower eyelid margin should be avoided. In general, lower fluences and fewer laser passes should be applied in the periorbital area to decrease the risk of lid eversion.
Hyperpigmentation is one of the more common side effects of cutaneous laser resurfacing and occurs to some degree in all patients with darker skin tones (Fig. 3).16,25 The reaction is transient, but its resolution can be hastened with the postoperative use of a variety of topical agents, including hydroquinone, retinoic, azeleic, and glycolic acid. Regular sunscreen use is also important during the healing process to prevent further skin darkening. The prophylactic use of these products preoperatively, however, has not been shown to decrease the incidence of post-treatment hyperpigmentation.13 Postoperative hypopigmentation, on the other hand, is often not observed for several months and is particularly difficult because of its tendency to be intractable to treatment. The use of an excimer laser or topical photochemotherapy to stimulate repigmentation has proven successful in some patients.66,67
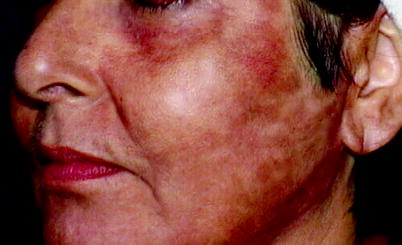

Fig. 3
Postinflammatory hyperpigmention following ablative laser resurfacing
Side effects and complications following Er:YAG laser irradiation are similar to those observed after CO2 laser treatment, but they differ in respect to duration, incidence, and severity.50,68,69 Although greater postoperative erythema is seen after modulated Er:YAG laser treatment than is usually produced with a short-pulsed Er:YAG system, the side effect profile and recovery period after modulated Er:YAG laser irradiation remain more favorable than after multiple-pass CO2 laser treatment. In an extended evaluation of 50 patients, investigators reported complete re-epithelialization in an average of 5 days after dual-mode Er:YAG laser skin resurfacing with only three patients having prolonged erythema beyond 4 weeks.68 In a split-face comparison of 16 patients after pulsed CO2 and variable-pulsed Er:YAG laser skin resurfacing, other investigators reported decreased erythema, less edema, and faster healing on the Er:YAG laser-treated facial half.70
Postinflammatory hyperpigmentation is not uncommon after any cutaneous laser resurfacing procedure. While hyperpigmentation following modulated Er:YAG laser skin resurfacing (mean, 10.4 weeks) can last longer than after treatment with a short-pulsed Er:YAG laser, it is not as persistent as that observed after multiple-pass CO2 laser resurfacing (mean, 16 weeks).69 However, when comparing the most current trends in ablative cutaneous laser resurfacing – single-pass CO2 versus multiple-pass, long-pulsed Er:YAG laser skin resurfacing – postoperative healing times and complication profiles are comparable, even in patients with darker skin phototypes. In a retrospective review and analysis of 100 consecutive patients, Tanzi and Alster44 showed average time to re-epithelialization was 5.5 days with single-pass CO2 and 5.1 days with long-pulsed Er:YAG laser resurfacing. Postoperative erythema was observed in all patients, lasting an average of 4.5 weeks after single-pass CO2 laser treatment and 3.6 weeks after long-pulsed Er:YAG laser treatment. Hyperpigmentation was seen in 46% of patients treated with single-pass CO2 and 42% of patients treated with the long-pulsed Er:YAG laser (average duration 12.7 and 11.4 weeks, respectively). Delayed-onset permanent hypopigmentation – a serious complication that has been observed several months after multiple-pass CO2 laser skin resurfacing – has not yet been reported following single-pass treatment. To date, only three cases of hypopigmentation after modulated Er:YAG laser skin resurfacing have been reported.71,72 Since it is possible for hypopigmentation to present several years postoperatively, clinical studies are ongoing to determine its true incidence following either single-pass CO2 or modulated Er:YAG laser skin resurfacing.
Non-ablative Laser Skin Resurfacing
Indications and Contraindications
Indications Mild facial rhytides, atrophic scars, and photo damage. Contraindications Patients with unrealistic expectations. Patients with darker skin tones (skin phototype IV–VI) have a high incidence of postoperative hyperpigmentation. Patients with perpetual sun exposure. Patients with history of herpes labialis may require prophylactic oral antiviral medications. |
Proper patient selection is critical to the success of non-ablative laser skin remodeling. Patients with mild-to-moderate facial photodamage or atrophic scars with realistic expectations of treatment are the best candidates for non-ablative procedures. Patients seeking immediate improvement in photodamaged skin or those who desire a dramatic result may be less than satisfied with the overall clinical outcome.
Techniques
Preoperative Management
Adequate preoperative patient evaluation and education. Oral antiviral prophylaxis in patients with history of herpes labialis. |
Adequate preoperative patient evaluation and education are absolute essentials (Table 4). For patients with a strong history of herpes labialis, prophylactic oral antiviral medications should be considered when treating the perioral skin. Reactivation of prior herpes simplex infection can occur after non-ablative laser treatment due to the intense heat produced by the laser or light source.
Table 4
Non-ablative laser resurfacing: patient selection, risks, and precautions
Preoperative patient evaluation |
|---|
Is the amount of photodamage amenable to non-ablative laser skin remodeling? Patients with mild-to-moderate facial photodamage are the best candidates for non-ablative procedures. Patients with severe rhytides and skin laxity may be disappointed with the overall clinical outcome |
Does the patient have a history of herpes labialis? Reactivation of prior herpes simplex infection can occur with perioral non-ablative laser skin modeling due to the intense heat produced by the laser. Patients with a strong history of herpes simplex labialis may require prophylactic oral antiviral medication to avoid a postoperative outbreak |
What is the patient’s skin phototype? Although the majority of current non-ablative systems used are within the mid-infrared range of the electromagnetic spectrum and not avidly absorbed by epidermal melanin, patients with darker skin phototypes may develop postinflammatory hyperpigmentation after non-ablative treatment. This temporary reaction may develop due to inflammation created by concomitant cryogen spray epidermal cooling |









