Stage
Description
Severity
0
Lymphostasis: a subclinical condition where swelling isn’t evident despite impaired lymph transport. It may exist for months or years before oedema occurs
None
I
An early accumulation of fluid relatively high in protein content. Oedema decreases with elevation. Pitting may occur. It may take up to a few hours of rest and elevation to reverse
Minimal (<20 % increase)
II
Pitting occurs, and the oedema is not appreciably reduced with elevation of the affected limb. In late Stage II, the tissue hardens and becomes fibrotic, and pitting no longer occurs
Moderate (20–40 % increase)
III
This stage is also referred to as elephantiasis. Pitting is absent. Skin changes like acanthosis, fat deposits and warty overgrowths may develop. Fluid may ooze from the skin due to high pressure in the lymphatic and venous vessels. It most commonly occurs in the legs and results from long-standing, inadequately treated or untreated lymphoedema
Severe (>40 % increase)
Preventive and therapeutic measures for the treatment of lymphoedema are listed below [3]:
Skin care is required to maintain good skin hydration and tropism and reduce the risk of infection.
Exercise promotes lymph flow and maintains good limb function.
Manual lymphatic drainage is a gentle skin massage that encourages lymph flow and is carried out by a trained therapist.
Support/compression with multilayer bandaging is applied to reduce the size and improve the condition of the limb to allow fitting of elastic compression garments, which when fitted correctly control swelling and encourage lymph flow. Compression garments should be worn while the patient is exercising to reduce lymphatic filtration.
Maintaining an adequate weight helps to prevent lymphoedema development, so dietary advice is important in all patients, but particularly in those who are overweight.
Lymphoedema of the arm without pain (lymphostasis) may be also due to massive axillary lymph node involvement, to scar tissue that cause blockages in lymph flow and make the enlargement of new lymphatic pathways harder and (rarely) to steroids used in some chemotherapy regimens that can cause fluid retention, usually temporary until the end of treatment.
Lymphoedema of the arm with pain or paraesthesia occurring several months after surgery may reflect recurrent tumour. This event is rare and the cancer is often not clinically discernible because it resides high in the apex of the axilla or lung and involves the brachial plexus. Patients may complain of tingling or pain in the hands and progressive weakness and atrophy of the hand and arm muscles. If sufficient time passes, a tumour mass becomes palpable in the axilla or supraclavicular fossa, but the patient is usually left with a paralysed hand unresponsive to therapy. These patients may receive RT to the axilla and supraclavicular fossa, if radiation has not previously been delivered to this region. The recurrence to the brachial plexus may not be easily seen on MRI or CT scanning. PET scanning may be useful in this circumstance. Occasionally, the pain of this nerve involvement is so severe that nerve blocks by a pain management specialist are necessary.
Range restriction of movement is assessed by the surgeon as active ranging at the shoulder joint, which was scored as equal to or decreased relative to the non-operated side.
Numbness and paraesthesia are evaluated by standard questions asked to each patient regarding the location and severity of the symptoms at the time of evaluation and compared with those in the postoperative period (unchanged, improved, significantly improved or completely resolved). Zones of persistent numbness, due to intercostobrachial nerve damage or section, should be outlined by the examining physician and confirmed by the patient.
Lymphatic cording, or axillary web syndrome, refers to one or more ropelike structures (more axillary strings characterise the web appearance) that develop mainly under the axilla (Fig. 21.1) but can extend to involve the medial aspect of the ipsilateral arm down to the antecubital fossa [5]. The syndrome usually appears after ALND, rarely after SLNB, 1–2 months after surgery of the axilla.
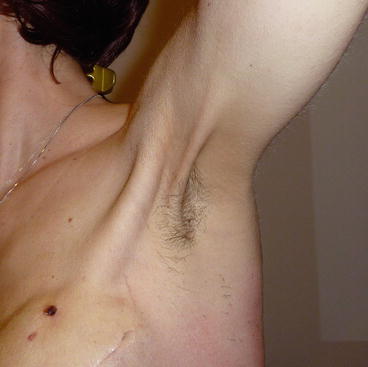

Fig. 21.1
The ropelike appearance of lymphatic cording (axillary web syndrome) visible under the left axilla of a 48-year-old woman, 2 months after she underwent lumpectomy and axillary dissection
Lymphatic cording is associated with pain and limitation of shoulder movement. Symptoms resolve in all patients usually in 2–3 months. The duration of symptoms did not appear to be shortened by nonsteroidal anti-inflammatory drugs, physiotherapy or active range of motion exercises. Physiotherapy is anyway recommended and usually involves a combination of gentle stretching of the cord, exercises and manual lymph drainage.
Chronic pain after breast surgery. Some women have problems with neuropathic pain in the chest wall, armpit, and/or arm after surgery that doesn’t subside over time. This is called post-mastectomy pain syndrome (PMPS) because it was first described in women who had mastectomies, but it can also happen after BCT. Studies have shown that about 20 % of women develop symptoms of PMPS after surgery. The classic symptoms of PMPS are pain and tingling in the chest wall, armpit, and/or arm. Pain may also be felt in the shoulder or surgical scar. Other common complaints include numbness, shooting or pricking pain, or unbearable itching.
PMPS is thought to be linked to damage done to the nerves in the armpit and chest wall during surgery, but the true causes are still unknown. Women who are younger, had an ALND, or who were treated with radiation after surgery are more likely to have problems with PMPS. PMPS can limit the use of the arm so that over time the woman could lose the ability to use it normally. Most women with PMPS say their symptoms are not severe. Severe PMPS (about 1 to 5 %) can be treated. Drugs commonly used to treat pain don’t always work well for nerve pain, but some new available narcotic-like medications, as pregabalin and tramadol, are effective and not as addictive as narcotics.
21.3.2 Gynaecological, Sexual and Reproductive Issues
Treatment may affect many aspects of sexuality, and BC survivors should be routinely questioned about concerns related to sexual health and counselled or referred as needed. Menopausal symptoms that result from chemotherapy (in premenopausal women) and hormonal therapy (regardless of menopausal status) may make sexual activity less enjoyable and even painful.
The psychological sequelae of a BC diagnosis can include strains on relationships and changes in body image, both of which can be detrimental to sexual functioning. Sexual dysfunction is associated with depression in BC survivors. It is important for physicians to routinely ask BC survivors about their sexual functioning. Referral to a sexual health and/or mental health expert may be helpful, and this issue is above all an investigational subject. Five questions facilitating discussion are the following.
Are you bound to be upset?
Is inability to relax persistent for more than half of the time?
Often women feel some change in their intimate relationship after BC diagnosis. How has this been for you?
People frequently feel some change sexually during this time. What have you noticed?
Speaking to a counsellor for a few sessions is often helpful because of the impact of BC diagnosis and its treatment on sexuality. Would you like a list of local counsellors?
Interventions for women who report dyspareunia or difficulty reaching orgasm may benefit from one or more of the following: local hormonal therapy, lubricants and counselling.
Local oestrogen therapy. Local oestrogen therapy comes in the form of creams and tablets, and it may provide relief of symptoms, but its use is controversial, particularly among women with a history of hormone receptor-positive BC. Although one study demonstrated that vaginal oestrogen results in a detectable increase in circulating oestradiol levels among women taking an aromatase inhibitor, another study suggests that vaginal oestrogen therapy does not result in an increased risk of recurrence in women taking endocrine therapy.
One study evaluating the impact of vaginal oestrogen on recurrence risk reported a low (less than 3 %) proportion of patients who used vaginal oestrogen (predominantly women taking TAM) and the finding that the use of vaginal oestrogen was not associated with an increased risk of recurrence. In light of this low-quality evidence, a decision to use vaginal oestrogens must be individualised, taking into account tumour characteristics, current symptoms and the risks and potential benefits of therapy, with input from both the primary oncologist and GP.
Topical lidocaine. For women with dyspareunia that appears to be isolated to tenderness at the vulvar vestibule, data from a prospective, randomised, double-blind trial suggest that topical lidocaine may provide relief. Treatment with topical aqueous 4 % lidocaine resulted in significant reduction in their worst pain score with intercourse and in no tenderness with speculum placement in almost all cases. Therefore, the data suggest that this intervention may be specific to women who have pain with penetration localised to the vulvar vestibule.
Vaginal dehydroepiandrosterone. Dehydroepiandrosterone (DHEA) is a steroid hormone produced by the adrenal glands. It is an indirect precursor to both oestrogen and testosterone, and levels decline with age. Vaginal DHEA preparations have been reported to improve vaginal health and sexual function in postmenopausal women. However, their safety and effectiveness in women treated for BC has not been cleared. A randomised multicentre trial examining the efficacy of adding dehydroepiandrosterone (DHEA) to a vaginal bioadhesive moisturiser in postmenopausal survivors of BC has found that daily rather than as-needed use of such a moisturiser significantly relieves symptoms of vaginal atrophy in these women and that when DHEA is added, survivors report significant improvements in sexual desire, arousal, pain and overall sexual function.
At 12 weeks, women treated reported improvements in sexual function and symptoms associated with vaginal atrophy. In addition, DHEA was associated with statistically significant improvements in sexual satisfaction as measured using standardised questionnaires. Compared with placebo, vaginal DHEA (at the 6.5 mg dose level) was associated with a higher incidence of voice changes and headaches, but well tolerated otherwise [6]. Although oestradiol levels were slightly increased by the vaginal DHEA in the study overall, this was not true in women receiving aromatase inhibitors. Although other studies are needed, current data support the use of vaginal DHEA as an option for postmenopausal BC survivors who suffer substantial vaginal complaints.
Ospemifene (Osphena®) is an oral selective oestrogen receptor modulator (SERM) acting similarly to an oestrogen on the vaginal epithelium, indicated for the treatment of dyspareunia. The FDA approved the medication in February 2013, but additional research addressing the expanded use of ospemifene in breast cancer patients is needed.
Fertility and pregnancy after BC. Young BC survivors may experience infertility after BC due to chemotherapy-related gonadotoxicity and the delay in childbearing required when women are taking the recommended 5 years of hormonal therapy.
For women with a history of BC, a subsequent pregnancy does not appear to compromise survival in patients with positive receptor status. A recent meta-analysis of 14 case–control studies that evaluated the impact of pregnancy on the overall survival of women with BC showed that BC patients who became pregnant had a 40 % reduction in the risk of death, compared with those who did not get pregnant. This outcome is likely explained by a selection bias known as the healthy mother effect, such that only healthy BC survivors were able to conceive and carry a pregnancy. However, other studies confirm pregnancy after BC is safe regardless of receptor status.
While some experts recommend that patients wait 2 years after completing adjuvant therapy before attempting conception in order to avoid pregnancy during the time of highest relapse risk, limited data suggest that pregnancy sooner is safe. Encouragingly, prior BC treatments do not appear to increase the risk of congenital malformation.
Contraception after BC. While women with a history of BC may wish to preserve fertility, they may not have an imminent desire to become pregnant. The safety and efficacy of hormonal contraception has not been well studied in women with BC, as these women have traditionally been excluded from studies of hormonal contraceptives. The levonorgestrel-releasing intrauterine device (LNG-IUD) has been evaluated in women with a history of BC, although primarily as a method of endometrial protection from the effects of TAM rather than as a contraceptive modality. Data are limited and inconclusive, but suggest an increased risk of cancer recurrence, although the difference was not statistically significant, despite a low rate of systemic hormone absorption.
World Health Organization guidelines for medical eligibility for contraceptive use suggest avoiding hormonal contraception in women with a current or past history of BC, particularly in those with hormone receptor-positive disease. Clinicians should discuss the use of a nonhormonal contraceptive method (condom, diaphragm, copper IUD) and help the woman choose the method most consistent with her lifestyle and beliefs.
21.3.3 Psychological and Relational Issues
Up to 30 % of women with BC develop an anxiety state or depressive illness within a year of diagnosis, which is three to four times the expected rate in matched community samples. After mastectomy without reconstruction, 20–30 % of patients develop persisting problems with body image and sexual difficulties. Breast-conserving surgery reduces problems with body image, but this may be offset by increased fears of recurrence.
After reconstruction some patients are predominantly worried about the satisfaction of their own needs and poorly sensitive to the attitudes and the reactions of the others. On the contrary, women with a strong personality face reconstruction as the conclusive symbolic action to overcome the illness and to repair the mutilation.
The same with regard to communication. A more practical division of psychological types is the Miller Behavioural Style Scale, where patients are divided into monitors or blunters. The former group of patients request much information, while the latter avoid asking questions. Yet, although some patients may demand information, they do not wish to be burdened with responsibility for any final decisions on treatment. Other patients express little interest in obtaining information about their disease or appear unreceptive to any information that is offered, either in verbal or written form.
Doctors can promote disclosure of such problems by asking questions and clarifying the responses about patients’ perceptions of the nature of their illness, their reactions to it and about their experience of losing a breast or having radiotherapy or chemotherapy. By being empathic, making educated guesses about how a patient is feeling and summarising what has been disclosed, doctors promote disclosure and expression of related feelings.
When there is any hint of anxiety or depression (Table 21.2), clinicians should inquire about key symptoms by asking open directive questions (What changes have you noticed while you have been depressed? How have you been sleeping?). Patients with problems with their body image should be asked how much they avoid looking at their chest wall and how they react if they catch sight of it. In patients with sexual difficulties, doctors should check whether they represent a new problem and explore the reactions of patients and their partners.









