Fig. 26.1
Clinical rectocele
Philosophy of Rectocele Repair: Transvaginal Versus Endoanal Perineal Repair
The gold standard for rectocele repair is still a matter of debate. The controversy concerning the anatomic importance of the rectovaginal septum and the extension of Denonvilliers’ fascia, the lack of correlation between the reported symptoms and the size of the rectocele, the role of associated pelvic floor abnormalities, and the small number of patients operated on in many specialist centers have largely precluded the accumulation of scientific evidence to define the best method for rectocele repair [1]. This is coupled with few well-constructed clinical trials in which different operative approaches have been used in matched patient cohorts [4, 5].
Up until the 1980s, rectocele was treated mainly by gynecologists with a conventional posterior colporrhaphy. The indication for surgery was, however, vaginal bulging and protrusion, and little attention was paid to associated bowel dysfunction [6, 7]. This procedure often was accompanied by a levatorplasty, believed to narrow the genital hiatus, as well as a variable incidence of sacrocolpopexy or culdoplasty designed to prevent attendant vaginal vault prolapse and coincident enterocele [8–10].
The recognition of the associated lower gastrointestinal symptomatology before and after rectocele repair became the province of the coloproctologist. Basically, surgery for rectocele is aimed at reinforcing the rectovaginal septum using different approaches, including combined transanal, transvaginal, transperineal, and laparoscopically assisted techniques. The most frequently practiced technique in the 1980s was the transanal approach described by various researchers, incorporating either definitive defect-specific repair of the rectovaginal septum or an anterior internal hemi-Delorme procedure [11–14]. With some minor differences, each operation consisted of the removal of the redundant mucosa and some form of muscular layer plication followed by suture of the remaining mucosa [15, 16] (Fig. 26.2).
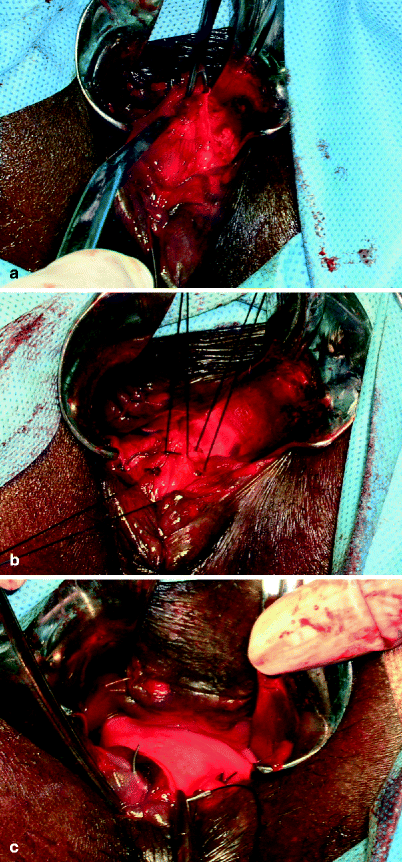

Fig. 26.2
Endorectal rectocele repair. (a) Mucosectomy. (b) Rectogenital septal repair and reinforcement. (c) Mucosal closure (Courtesy of Professor A.P. Zbar)
A full-thickness, unselected, mucosal and muscular plication without resection was proposed by Block [17] as an imbrication or inversion suture/rectocele obliteration. Block claimed a high success rate, although many of the patients had relatively asymptomatic small rectoceles. The endorectal approach has evolved, with the use of a dedicated stapler, to comprise the stapled transanal rectal resection (STARR) and, more recently, the trans-STARR contour-stapled technique, both of which have been proposed with some caution [18, 19]. These procedures have been extensively used in Europe in the past few years, with high success rates reported, although the mechanism of action of the neorectum is unclear as are many of the relative contraindications for use, resulting in a dual dialogue. Some groups have reported significant neosyndromes after the STARR procedure that are somewhat resistant to medical rehabilitative and surgical therapies. Furthermore, great caution needs to be exercised in those patients presenting with a rectocele who complain of urgency or frank incontinence because up to one third of patients after a STARR procedure may complain of troublesome, long-lasting incontinence and urgency [20]. One of the absolute contraindications to the STARR procedure was combined enterocele plus rectocele that has been obviated to some extent by the use of a laparoscopically guided STARR operation [21]. The issue of the controversial nature of the STARR procedure and its descendants is discussed in Chap. 27.
An alternative approach is via the perineum. After a skin or vaginal incision is made, the rectum is separated from the vagina up to the top of the rectocele and its muscular layer is reinforced by direct plication or with a range of mesh types. Currently, the transvaginal approach is used less frequently and is performed mainly by gynecologists if there is concomitant vaginal pathology that requires a vaginal incision, namely, a vaginal vault prolapse or a concomitant cystocele. The transvaginal rectocele repair with mesh reinforcement commences with a midline vertical incision over the posterior vaginal wall. If a perineorrhaphy is required, a diamond-shaped incision of the perineal skin and excision of the diamond-shaped tissue are performed. The vagina is then mobilized from the rectovaginal septum. The rectovaginal fascia is plicated and a strip of mesh is placed over the rectovaginal septum. The role of an attendant levatorplasty is controversial. The vaginal mucosa is closed over the mesh [22, 23]. In an attempt to further reduce the recurrence rate, combined transanal and posterior vaginal repair has been employed.
Recently, a more complex approach has been advocated via a laparoscopic ventral rectopexy after careful dissection of the rectovaginal space from the Douglas pouch [24]. The approach provides nerve-preserving rectovaginal support with the additional use of perineal mesh, rectal/levator fixation, levatorplasty, and a mesh sacropexy [25, 26], with good initial results, although the patient groups so far reported have been fairly heterogeneous. Despite its advantage when dealing with coincidental anorectal diseases, no significant difference has been found between the transanal approach and traditional posterior colporrhaphy with regard to constipation, anal incontinence, or dyspareunia, although as stated earlier the literature comparing the two different approaches is scarce. In the transperineal approach, a transverse perineal incision is made and the plane between the external anal sphincter and the posterior vaginal wall is developed up to the vaginal apex to expose the rectocele, the perirectal fascia, and the medial margins of the levator plates. A variety of both synthetic and nonsynthetic graft materials have been used in rectocele repair to enhance anatomical and functional results and to improve long-term outcomes. After this site-specific repair, four to five delayed absorbable or nonabsorbable sutures are placed in the medial aspect of the levator plate laterally on each side, from the vaginal apex toward the perineal body. [22, 27]
Any of these approaches to reoperative surgery is subject to some degree of failure where it is dependent upon the prior surgical technique used, the extent of the recurrence, and the patient’s motivation to undergo further surgery. Reoperation is always difficult and risky, and the most challenging situations are caused by the presence of nonresorbable suture material, particularly staples or synthetic meshes previously deployed in the rectovaginal space.
Reasons for Failure
The literature concerning the outcome of reoperation for failed rectocele is very scarce, and a distinction should be made between anatomical failures and functional failures: the latter occur despite an anatomical correction of the rectocele. Anatomical failure (persistence) of the rectocele is usually due to an inappropriate surgical technique and is more frequent in large pulsion rectoceles, often secondary to hysterectomy. Functional failure of the rectocele repair is usually due to an undetected rectoanal intussusception or other associated abnormalities of the pelvic floor, including a sigmoidocele, perineal descent, and hyposensitivity of the rectum. The use of liquid barium instead of dense barium paste mimics normal feces during defecography, missing oral contrast medium for the small bowel and anorectal manovolumetry in the diagnostic work-up can be as possible for this failure. [28].
Although rectocele size, barium entrapment, and the need for vaginal digitation often are used to select patients for surgery, there is minimal evidence that these factors affect the overall clinical outcome after repairs [29]. In this respect, Infantino et al. [30] showed that restoration of the anatomy did not always lead to restoration of function. For these patients, an intense preoperative work-up including a psychological interview with detailed anamnestic data incorporating a history of child abuse must be sought. The other common cause of failure is an inadequate or incorrect surgical choice, and partial repair or early breakdown of the sutures can account for many failures of rectocele repair. A third cause of failure is lack of patient satisfaction despite a normalized proctogram and the “normalization” of bowel motion frequency; anorectal symptoms attributed to a rectocele overlap with the clinical features of other pelvic floor, anorectal, and colonic disorders often can coexist in the same patient [31]. Recent data also have shown that many patients presenting with rectocele and typical obstructed defecation syndrome also have symptoms consistent with a diagnosis of irritable bowel syndrome (or they revert to these main symptoms during follow-up), suggesting that such cases may find less functional benefit in definitive rectocele repair [32, 33]. Vaginal or perineal digitation is thought to be specific for an operatively curable rectocele [34]; however, sometimes even these symptoms may improve with dietary changes or biofeedback training or may even spontaneously resolve over time. Similarly, the feeling of incomplete emptying may occur from an irritable rectum or a coincident enterocele, sigmoidocele, or rectal intussusception, despite complete rectal evacuation [35]. In other cases, some women with descending perineum syndrome may have difficulty evacuating in the absence of a discrete rectocele. In this respect, the postoperative criteria of success are often inconsistent and poorly defined [36–38].
Management Guidelines for Failed Rectocele Repair
The surgical salvage options for recurrent rectocele largely depend on the nature and conduct of the previous operation. The most common clinical scenarios are presented below, and an algorithm of this approach is presented in Fig. 26.3.
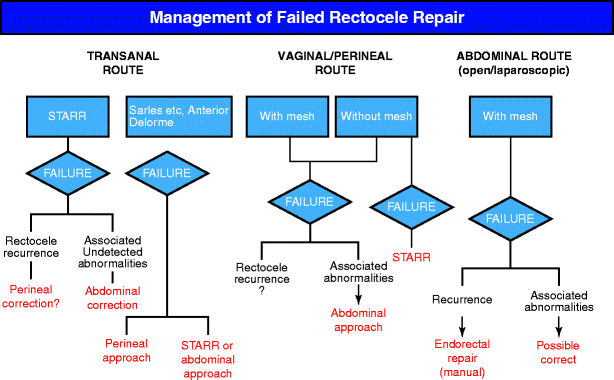

Fig. 26.3
An algorithm approach toward failed rectocele repair. Management is dependent upon initial surgical type. STARR stapled transanal rectal resection
Failed Rectocele Repair after Transanal Approach
Patients treated with any type of mucosectomy and hand-sewn rectal plication presenting with persistence of rectocele-related symptoms can easily be reoperated upon via the same route using either a stapled or manual mucosectomy, with complete healing of the previous wounds provided that the existence of other pelvic floor functional disturbances have been excluded. An abdominal approach is also feasible, according to the surgeon’s preference [31]. By contrast, a repeat rectocele repair after the STARR procedure has a higher risk because of the presence of scarring and staples retained in the anterior rectal wall [18]. A perineal instead of a transanal approach is preferred in these cases, although separation of the vagina from the stapled anterior rectal wall may be quite difficult and bloody.
Failed Rectocele Repair after Perineal/Vaginal Approach
Usually the performance of a new operation for rectocele using the same perineal route used in an earlier surgery is quite difficult, particularly when a nonresorbable mesh has been used; there is an attendant risk of rectovaginal fistula. In many of these cases it is virtually impossible to separate the rectum from the vagina, and rectal perforation in the presence of a foreign mesh is a further major risk. If a previously undetected pelvic floor abnormality could explain the symptom persistence, it can be treated differently [31]. When a rectocele recurs after a simple plication of the rectal wall hand-sewn with absorbable sutures, a transanal approach—even with a STARR procedure—may be considered.
Failed Rectocele Repair after Abdominal Approach
Finally, a rectocele can recur after an abdominal approach (laparoscopic or open). Usually nonresorbable mesh has been used to reinforce the rectovaginal septum as well as to obliterate the pouch of Douglas (a Moshcowitz procedure) and create a sacropexy. In these cases, reoperation should be performed via a different route, possibly by endorectal means, because remobilization of the rectum abdominally has a high risk of perforation with the added concern of sometimes catastrophic sacral bleeding in the presence of dense adhesions from a previous sacropexy. The use of absorbable, biologic meshes has been proposed to reduce this risk, although little is known about their long-term efficacy [39].
Repeat Rectocele Surgery: The Transperineal Approach
Filippo Pucciani3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








