and Veronica Tomasello2
(1)
Department of Plastic Surgery and Burns, University Hospital Vall d’Hebron, Barcelona, Spain
(2)
Cannizzaro Hospital, Catania, Italy
Abstract
Reconstructive allotransplantation has emerged as the ultimate restorative technique for treating face deformity, hand amputations and others. With the development of novel, more effective, immunosuppressant regimens, which shall decrease the advent of toxic side effects, the indication for this new technique may widen. In fact, the achievement of such a regimen that minimised side effects and counterbalanced the ethical issues in reconstructive allotransplantation would allow for the transplantation and restoration of any anatomical and functional unit of the human body. Cell therapy, tissue engineering and new synthetic polymers will help for the development of a true restorative surgery in the future, combining the knowledge and expertise of transplantation medicine with the advent and development of biological and synthetic tissue engineering.
Keywords
SurgeryReconstructionTransplantation processAnastomosisImmunosuppression14.1 Introduction
Reconstructive allotransplantation has emerged as the ultimate restorative technique for treating face deformity, hand amputations and others. With the development of novel, more effective, immunosuppressant regimens, which shall decrease the advent of toxic side effects, the indication for this new technique may widen. In fact, the achievement of such a regimen that minimised side effects and counterbalanced the ethical issues in reconstructive allotransplantation would allow for the transplantation and restoration of any anatomical and functional unit of the human body. Cell therapy, tissue engineering and new synthetic polymers will help for the development of a true restorative surgery in the future, combining the knowledge and expertise of transplantation medicine with the advent and development of biological and synthetic tissue engineering (Table 14.1).
Table 14.1
Common principles of modern regenerative/restorative surgery
Cell therapy |
Preservation and tissue modulation |
Wound matrices and biomaterials |
Immunological interaction |
Engineered matrices and constructs |
Transplantation medicine/immunomodulation principles |
The surgical treatment of face transplant patients follows the same principles and tenets observed in composite tissue transfer (free flaps) and craniofacial/maxillofacial surgery. A thorough study of the deformity and the health status of the patient is essential to make a global plan of treatment. All steps of the operation must be outlined, taking into consideration all difficult phases and creation of a strong multidisciplinary team. It is recommended that the recipient’s operations be prepared and performed beforehand in the anatomy dissection room in order to avoid mistakes and unexpected errors during the operation. The surgical team should prepare all details in order to be prepared for a continuous flow during the transplantation procedure. All surgical team members and anaesthesiologists must be well versed and have a complete knowledge of the whole procedure, since rotation of human resources during the transplantation phase is necessary to maintain safety during the surgical intervention. The main principles of face transplantation include the following:
Organisation of the operating room and human resources
Patient’s preparation and induction therapy
Extirpation of recipient’s tissues
Revascularisation
Face transplantation
Closure and patient’s transfer
14.2 Organisation of the Operating Room and Human Resources
Large and spacious operating rooms are recommended for reconstructive allotransplantation. In general terms, rooms exceeding 40 square metres are advised. During face transplantation, a large number of transplantation teams are working together at the same time. It is not uncommon to join together at any given time two anaesthesiologist, two scrub nurses, four surgeons and two circulating nurses. Enough working space is necessary, to accommodate human resources and the necessary equipment (microscope, anaesthesia carts, instrumentarium, blood requirements, craniomaxillary instruments, etc.; see Fig. 14.1).
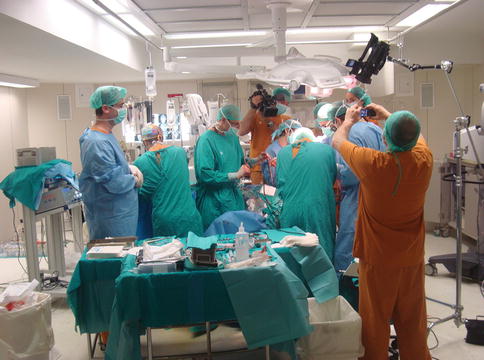

Fig. 14.1
Large and spacious theatre operating rooms are necessary for face transplantation. Microvascular instruments, microscopes, craniomaxillofacial instruments and others are common instruments during this type of procedures
As soon as the team is warned that the transplant may be imminent, the head nurse should be informed in order to make ready all surgical instruments and blood products, prepare the OR nurse team and organise the shifts. It is advised to organise the team with two scrub nurses per shift and rotate them every 2–3 h. A total of 3–4 nurses may be necessary to provide enough rest during the procedure. In addition, the nursing team is completed with enough circulating personnel to provide external support.
Quick and thorough preparation of the anaesthesiology team is performed simultaneously. The head anaesthesiologist is informed of the proposed transplant procedure in order to make the team and the necessary material available. It is recommended that a minimum of two anaesthesiologists are present in the operating theatre at any given time. Major bleeding and difficult airway management is expected, and prompt decisions and manoeuvres may be necessary. In our experience, the anaesthesiology team should be completed with a third anaesthesiologist (minimum) in order to provide the necessary rest for the team. It is not uncommon for face transplantation procedures to last between 18 and 30 h. Under these circumstances, it is mandatory to rotate team members in order to maintain a safe environment and provide a good progress of the operation.
The surgical team should be formed by team players that are readily available all year round. Face transplantation is an urgent procedure that is carried out any time when a compatible multiple-organ donor is approached and informed consent from relatives is obtained. In comparison to other solid organ transplantation programmes, it is not feasible to have an on-call team on a 24-h basis, since donors are not commonly available and the number of patients in the waiting list for VCA is very low. Therefore, it is extremely important to build a strong team of enthusiastic surgeons that shall guarantee the successful development of a VCA procedure. Once the operation is planned, the team of surgeons should be organised in shifts, similarly to anaesthesia and nursing teams. Our approach consists of three to four plastic surgeons per shift (depending on the complexity of the phase of the operation) that rotate every 2–3 h (Table 14.2). The team leader takes the responsibility to organise all rotations and it is his/her responsibility to oversee the good progression of the operation. It is advisable to name an assistant team leader in order to provide the necessary rest for the team leader at any given time. The master plan for the operation should be outlined before the patient is taken to operation room and all difficult points revised.
Table 14.2
Recommended rotation of team members
Anaesthesia: |
2 anaesthesiologists on site; rotate every 3–4 h |
Minimum of 3 anaesthesiologists |
Surgeons: |
3–4 surgeons on site; rotate every 2–3 h |
Minimum of 6–8 surgeons |
Scrub nurses: |
2 scrub nurses; rotate every 2–3 h |
Minimum of 3–4 nurses |
The head anaesthesiologist, head nurse and team leader must revise and make sure that all necessary equipment, materials, blood products and necessary support from other services are ready and available before the recipient is taken to the operating room. Other necessary and mandatory team members include transplant coordinators (at least one coordinator should be present during the whole procedure), infectious diseases/immunologists and transplant surgeons (these team members shall help in infectious disease prophylaxis, induction immunosuppressant therapy and technical pitfalls during the transplantation). It is not absolutely necessary that they are present in the operating room, although it is mandatory that they can be reached within minutes. Other support services include porters, housekeeping, ward nurses, pharmacy, mass media coordination and tissue/blood banking. The rest of the team members (Table 14.3) should be informed of an imminent transplant since their assistance will be necessary from day 1.
Table 14.3
Organisation of face VCA team
1. Chief surgeon (team leader) |
2. Plastic surgeons |
3. Transplant surgeons |
4. Transplant coordinators |
5. Immunologist |
6. Infectious disease specialist |
7. Psychiatrist |
8. Psychologist |
9. Pathologist |
10. Nephrologists |
11. Surgical critical-care specialist |
12. Nurses |
13. Mass media relations coordinators |
14. Rehabilitation services |
15. Speech pathologist |
16. Dietician |
17. Pharmacists |
18. Support services |
The organisation of the operating room within the hospital differs depending on the type of the expected donation. If the donor is located in the same hospital, our advice is to follow the Boston approach if possible: the donor’s and recipient’s operating rooms should be run in parallel. With this approach, the graft is procured in the operating room next door, and it can be transported within seconds to the recipient’s room (which is normally located a few metres on the room next door). With this organisation, complex and cumbersome packaging in a container with preservation fluid is avoided and the graft is manipulated as any other routine free flap. Once the VCA graft is detached from the donor (after preservation fluid infusion), the graft is transported to the recipient’s site in the standard fashion. If this approach is selected, one must remember, though, that a third parallel room (on the other side of the donor’s theatre) may be required to perform bank surgery on solid organs (Fig. 14.2). This approach, when feasible, saves time and helps with the organisation and coordination of both the donor’s and the recipient’s transplant teams. On the other hand, if the donor is not located in the same hospital or, given the architecture of the hospital (commonly in health science centres, when different hospitals form the complex), the donation must be contemplated as a distant donor, both the donor’s and recipient’s theatres being distant and the transportation of the VCA graft must be performed in a container with preservation fluid and secured and transferred to the recipient’s operation room as in any standard solid organ transplantation procedure. When this approach has to be implemented, cold ischaemia time should be kept to a minimum and the organisation and coordination of both teams is much more complicated. Direct communication between both theatres’ teams is essential to couple both surgeries; as soon as the procurement process is considered to progress in good condition, the recipient’s team should start the procedure to induce the immunosuppressant protocol, secure the airway and prepare the recipient’s vessels.


Fig. 14.2
Common organisation of parallel operating rooms for VCA procedures. Simultaneous operating rooms may be added in case transplantation of solid organs is planned in the same institution
14.3 Patient Preparation and Induction Therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








