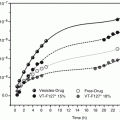
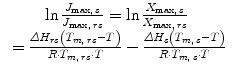Fig. 12.1
Relationship between the steady-state flux of lidocaine and prilocaine through regenerated cellulose membrane and their solubility-normalized ratio in phosphate buffer solution: prilocaine (♦), lidocaine (□). Each point represents mean ± 1 standard deviation (n = 5). A linear trend was observed between the steady-state flux of prilocaine and lidocaine and their solubility-normalized ratios in the donor solution and was plotted (represented by black line) (Reproduced with permission from Fiala et al. (2008))
Relating the correlation observed for lidocaine and prilocaine between the normalized drug ratio in the applied bulk solution and the species transport back to the principles of the Higuchi equation allows the reduced diffusion of one agent in the presence of the second to be predicted by the initial composition of the applied saturated solutions using Eq. 12.1, where the rate of transport for an individual molecule (dq/dt) was related to the membrane surface area (A), the membrane diffusion coefficient (D), the thermodynamic activity (α), the activity coefficient in the membrane (γbar), the diffusion path length (L), and the normalized ratio of the applied agent (N):
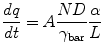
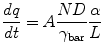
(12.1)
In the case of lidocaine and prilocaine combinations, the normalized solubility was calculated using Eqs. 12.2 and 12.3, respectively:
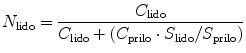
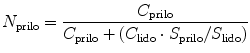
where S prilo, S lido was defined as the solubility of prilocaine and lidocaine in individually saturated solutions and C prilo, C lido the concentrations of prilocaine and lidocaine in the application solutions. The self-diffusion coefficient measurements (which do not change upon the mixing of lidocaine and prilocaine at equimolar ratios ~ 7.5 × 10 −6 cm2/s) were cited in the paper by Fiala et al. (2008) to suggest the trends in the data were due to a reduction in the capacity of the membrane to allow unhindered diffusion of one compound in the presence of the second and not drug-drug interactions (Nyqvist-Mayer et al. 1986).
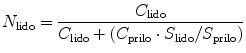
(12.2)
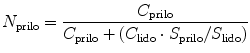
(12.3)
12.3 Dual Drug Partitioning
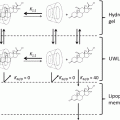
Building the effects of drug partitioning into the dual drug transport process is very important in percutaneous penetration. The partition coefficient (log P) of a molecule is typically measured as a ratio between the affinity for a standard oil phase (often octanol) and water. It can be used to predict the ability of a molecule applied to a hydrophobic barrier to pass into it. In terms of the human skin, if the log P is high for a particular drug, it will move across the first energy barrier created by the water/SC interface and into the outer layers of the skin. However, when a polar vehicle is used to administer a compound to the skin’s surface, the hydrophobic SC is sandwiched between two hydrophilic phases. The epidermal tissue underlying the SC contains more water and fewer lipids than the skin’s outermost layer, and as a consequence, it is distinctly more hydrophilic than the SC (Scheuplein and Blank 1971). Therefore, even if an agent applied topically to the surface of the skin passes into the SC, its subsequent transport through the tissue into deeper layers may be retarded by a second energy barrier (Williams and Barry 2004). As a consequence, a more detailed understanding of the transport process can be obtained if the effects of partitioning into a confluent barrier from a specific application vehicle are recorded and compared to predicted values using transport models that employ log P rather than relying on the latter alone. Human skin can be used in such studies, but it suffers from the problem of structural breakdown when long equilibration times are required to gain the measurements. Silicone membranes can be a useful alternative to human skin in partitioning experiments. Many types of silicone membranes do not have the same structural stratifications as skin, but they form a continuous hydrophobic barrier that is relatively inert and immiscible with polar solvents. They can be sandwiched between two water phases using a Franz cell setup in vitro; thus, they can generate transport and partitioning data using equivalent experimental parameters.
Transport studies using binary mixtures of lidocaine and prilocaine dissolved at different ratios in water (at a pH that retains both agents in the unionized state) have shown that the linear relationship between drug transport and normalized applied concentration observed in the RCM studies (Fig. 12.1) was not conserved when the barrier was switched to silicone (Fig. 12.2). The reason for this was assigned to the changes that occurred during the act of partitioning, which for the confluent silicone membrane, unlike the porous RCM, was predicted to have a significant effect on the transport process (Fiala et al. 2008). The data reported by Fiala et al. (2008) indicated that prilocaine partitioning remained unchanged irrespective of the concentration of lidocaine present in the binary drug mixtures, while lidocaine partitioning was enhanced when increasing amounts of prilocaine were present in the applied solutions. The solubility parameters of silicone, lidocaine, and prilocaine are 7.3 (cal cm−3)1/2, 10.68 (cal cm−3)1/2 and 11.05 (cal cm−3)1/2, respectively (Fedors 1974). According to these values, the presence of either prilocaine or lidocaine in the membrane could theoretically alter the solubility parameter of silicone in a manner which may facilitate the passage of the second agent into the barrier when a binary mixture was applied onto its surface. Experimentally, a more efficient partitioning was only observed for lidocaine during the dual drug application process (Fiala et al. 2008). This is difficult to logically explain using the standard transport theories outlined in the literature. Furthermore, the transport data generated by Fiala et al. (2008) suggested that influence of the dual drug application on partitioning was relatively minor in comparison to drug diffusion when an aqueous application vehicle was used. These findings are not in agreement with other published work that has suggested eutectic system enhancement of drug delivery to the skin was mediated through changes in the partitioning process (Benson 2005; Stott et al. 2001). This raises a question as to the particular context in which the previous hypothesis regarding eutectic penetration enhancement was generated and the generality of the conclusions from any of the aforementioned studies.
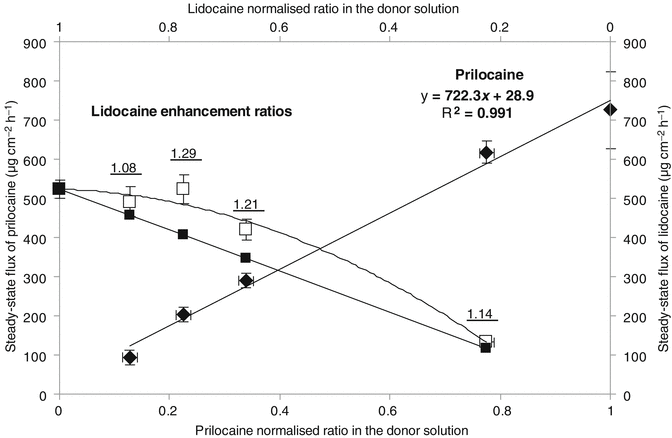

Fig. 12.2
Relationship between the steady-state flux of lidocaine and prilocaine through silicone membrane and their solubility-normalized ratio in phosphate buffer solution: prilocaine (♦), lidocaine (□). Theoretical lidocaine flux (■) was calculated assuming that the diffusion volume was changing as a function of the normalized ratio. Each point represents mean ± 1 standard deviation (n = 5). Enhancement ratios of lidocaine were calculated as the ratio of the actual to the theoretical steady-state flux and are indicated by the numbers on the graphs (Reproduced with permission from Fiala et al. (2008))
12.4 Eutectic Combinations
Previous literature has suggested that the skin penetration enhancement effects of eutectic systems can be attributed to superior solubility of the active molecules presented by theses systems in SC lipids (Alexander et al. 2012; Kaplun-Frischoff and Touitou 1997). This has been linked to the “melting point theory” which suggests that agents with a lower melting point (a key characteristic of all eutectic systems) will often have a greater propensity to associate with solvent molecules (Benson 2005). It has also been suggested in the literature that once the agents delivered by eutectic systems are within the skin, the co-localization of the diffusing species aids barrier penetration by interacting and modifying the skin structure (Watanabe et al. 2009; Woolfson et al. 2000). The facilitation of transport by eutectic systems through drug-skin interaction seems logical from both the perspective of increasing drug partitioning into the barrier and facilitating the movement of drugs through the barrier as such effects seem to be similar in nature to when the skin barrier is heated (Stott et al. 1998). According to Wood et al. (2012), heating the skin has a greater consequential effect on the process of drug partitioning compared to drug diffusion, but a similar conclusion is difficult to substantiate for eutectic systems. Both Pugh et al. (1996) and Fiala (2008) suggest that eutectic systems may have a negative influence on drug diffusion due to species competition and interaction in the solution state. This raises a question as to how, in the context of a topical formulation applied to the surface of the skin, the issues of competitive transport, molecular interactions, and facilitative partitioning function to generate the final transport rate generated from different eutectic systems. In an attempt to provide a critique on this matter, there is a need to highlight the specific details of how the various hypotheses surrounding the eutectic systems have been derived.
One model that relates melting point to SC solubility was proposed in the 1980s by Kasting et al. who suggested a relationship between transdermal flux and melting point of the drug based on the concept of ideal solution state chemistry (Kasting et al. 1987). Using this model, the ideal solubility of a drug (S ideal assuming thermodynamic ideality in terms of intermolecular interactions) in the skin lipids can be obtained using Eq. 12.4:
![$$ {S}_{\mathrm{ideal}}=\frac{\rho }{1-\left\{1- \exp \left[\frac{\varDelta {S}_f}{RT}\left({T}_m-T\right)\right]\right\}\frac{M_1}{M_w}} $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_12_Chapter_Equ4.gif)
where ρ is the density of the skin lipids, M 1 is their average molecular weight, M w is the molecular weight, T m is the melting point in degrees Kelvin, and ΔS f is the entropy of fusion of the drug. The ideal solubility was then used to predict a maximum flux, J m (Eq. 12.5):
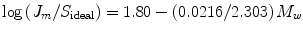
where M w is the molecular weight. ΔS f shows limited change as a function of melting point, but S ideal increases exponentially with decreasing melting point for any given molecular weight, and this relationship is thought to drive flux across the skin, which would also theoretically increase exponentially. Touitou et al. (1994) used melting temperatures as indices to predict the relative transdermal fluxes of a series of enantiomeric eutectic mixtures. Solubility, expressed as solute mole fraction (X) in a given solvent, was related to the melting temperature (T m) and the enthalpy of fusion (ΔH) using Eq. 12.6:
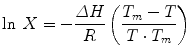
![$$ {S}_{\mathrm{ideal}}=\frac{\rho }{1-\left\{1- \exp \left[\frac{\varDelta {S}_f}{RT}\left({T}_m-T\right)\right]\right\}\frac{M_1}{M_w}} $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_12_Chapter_Equ4.gif)
(12.4)
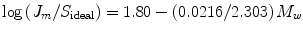
(12.5)
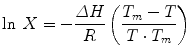
(12.6)
From this calculation, the maximum fluxes of one pure enantiomer (J max, s) compared to the racemic mix (J max, rs) were predicted (Eq. 12.7):