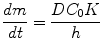Fig. 2.1
Transmission electron micrograph of several epidermal lamellar granules. Bar = 200 nm
Since lamellar granules are lipid-rich, they have a low buoyant density. This unique property has been exploited to isolate lamellar granules (Freinkel and Traczyk 1985; Wertz et al. 1984; Grayson et al. 1985; Madison et al. 1998; Sando et al. 2003). Direct chemical analyses of isolated lamellar granules (Freinkel and Traczyk 1985; Wertz et al. 1984; Grayson et al. 1985) have shown that these organelles are rich in phosphoglycerides, sphingomyelin, glucosylceramides, and cholesterol. Fatty acids, cholesterol esters, and ceramides were minor lipid components. The most abundant of the glucosylceramides is a structurally unusual linoleate-containing acylglucosylceramide (Wertz et al. 1984; Madison et al. 1998). This consists of 30- through 34-carbon ω-hydroxyacids amide-linked to sphingosine (and dihydrosphingosine) with linoleate ester-linked to the ω-hydroxyl group (Wertz and Downing 1983; Abraham et al. 1985). Linoleate has long been known to be required for proper formation and maintenance of the permeability barrier of the skin, and it has been proposed that this unusual sphingolipid is directly involved in this requirement (Wertz and Downing 1982). Based on extensive electron microscopic studies, Landmann has proposed that the internal lamellae of the lamellar granules are stacks of flattened lipid vesicles (Landmann 1986). It was proposed that in assembly of these stacks of flattened vesicles, the long ω-hydroxyacyl chain of the acylglucosylceramide completely spans one region of bilayer, while the linoleate inserts into an adjacent region of bilayer, thus riveting the two sections of bilayer together at a molecular level. In support of this suggestion, it was shown that acylglucosylceramide does cause flattening and aggregation of synthetic lipid vesicles. Lamellar granules and acylglucosylceramides have been found in keratinizing oral epithelium, but neither the organelle nor the unusual sphingolipid are present in nonkeratinizing oral epithelium or other tissues. Likewise, lamellar granules and acylglucosylceramides have been found in epidermis of a number of terrestrial mammals, birds, and reptiles, but both are absent from epidermis of fish and amphibians, where there is no stratum corneum.
The hydrolytic enzymes delivered to the intercellular space by lamellar granules, in general, have low pH optima like lysosomal hydrolases (Freinkel and Traczyk 1985; Wertz et al. 1984; Grayson et al. 1985; Madison et al. 1998). The enzymes detected include acid phosphatase, aryl sulfatase, galactosidase, galactosaminidase, glucosidase, glucosaminosidase, phospholipase A, sphingomyelinase, carboxypeptidase, cathepsin B, acid lipase, and ceramide glucosyltransferase. Of these enzymes, the specific activities in the isolated lamellar granules were lower than found in crude homogenates for arylsulfatase (both A and B) as well as sterol sulfatase (Grayson et al. 1985). The lipid-hydrolytic enzymes are important in converting the initially extruded lamellar granule lipids into the ceramide, cholesterol, and fatty acid mixture that is found in the intercellular spaces of the stratum corneum. It is this intercellular lipid that determines the permeability barrier of the skin, under normal circumstances (Wertz 2000).
2.3 Intercellular Lamellae
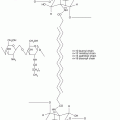
Among the enzymatic actions that occur at the boundary between the granular layer and the stratum corneum are the deglycosylation of glucosylceramides and the hydrolysis of sphingomyelin to produce ceramides. Representative structures of the ceramides found in porcine stratum corneum are presented in Fig. 2.2, along with the ceramide nomenclature system proposed by Motta and colleagues (Wertz and Downing 1983b; Motta et al. 1993). In the Motta nomenclature system, one letter is used to indicate the type of fatty acid (N for normal fatty acid, A for α-hydroxyacid and O for ω-hydroxyacid), one letter is used to indicate the long-chain base (S for sphingosine, P for phytosphingosine, and H for 6-hydroxysphingosine), and the presence of an ester-linked fatty acid is indicated by a prefix E. Thus, the ceramide-containing saturated fatty acids amide-linked to sphingosine bases can be designated as Cer[NS]. The unusual ceramide at the top of Fig. 2.2 is an acylceramide or Cer[EOS]. Most of the ceramides are cylindrical in shape, which favors formation of highly ordered, and thereby impermeable, membrane domains. The unusual Cer[EOS] is thought to play a central role in formation of 13 nm trilaminar lipid structures (Madison et al. 1987; Kuempel et al. 1998; Groen et al. 2010). In these trilaminar structures, it has been proposed that the ω-hydroxyacyl portion of Cer[EOS] spans the outer layers, while the linoleates insert into the central lamella (Hill and Wertz 2003). With this arrangement, the outer two lamellae are highly saturated, while the central lamella contains all of the double bonds from the linoleate chains. This stabilizes the trilaminar structures into 13 nm units that have been seen using transmission electron microscopy with ruthenium tetroxide fixation (Madison et al. 1987; Kuempel et al. 1998) and with X-ray diffraction (Groen et al. 2010). One consequence of this arrangement is that the central lamella will reduce more ruthenium than the outer lamellae. This results in alternating broad-narrow-broad lucent bands in the electron micrographs, as can be seen in Fig. 2.3.
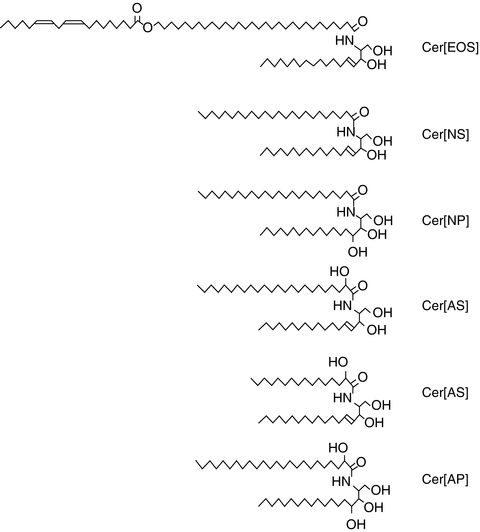
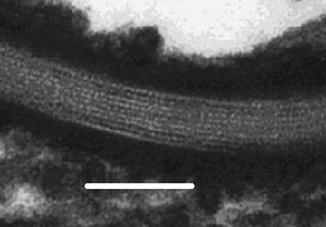


Fig. 2.3
Transmission electron micrograph of intercellular lamellae in the stratum corneum. Bar = 80 nm
The lateral packing of the lipids in normal human stratum corneum is predominantly orthorhombic (crystalline) with small amounts of hexagonal packing (gel) (Pilgram et al. 1998; de Jager et al. 2004). In some skin diseases in which lipid composition is altered and barrier function is diminished, such as atopic dermatitis and lamellar ichthyosis, the hexagonal phase increases relative to orthorhombic phase (Pilgram et al. 2001).
2.4 Covalently Bound ω-Hydroxyceramide
It is thought that approximately two-thirds of the acylglucosylceramide molecules associated with the lamellar granules are present in the bounding membrane with the glucosyl moiety at the inside surface of the granule (Wertz 2000). When the bounding membrane of the lamellar granule fuses into the cell plasma membrane, this inverts the orientation of the acylglucosylceramide. The glucose is then removed, and two stereoselective lipoxygenase attacks on the linoleate chain precede its removal and transfer of the resulting ω-hydroxyceramide to the outer surface of the cornified envelope (Zheng et al. 2011). The attachment of the ω-hydroxyceramide to the cornified envelope is through ester-linkages that may be produced through the action of a transglutaminase (Nemes et al. 1999). Thus, the stratum corneum becomes an array of flat keratin-filled cells bounded by a cornified envelope with a monolayer of covalently bound lipids on the outer surface embedded in a multilamellar array of free lipids.
2.5 Penetration Pathways
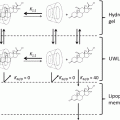
In a classic review, Scheuplein and Blank considered potential pathways through which small molecules could pass through the stratum corneum (Scheuplein and Blank 1971). These included a paracellular route through the intercellular spaces, a transcellular route, follicular penetration, and entry through the sweat ducts. For human skin, they argued that entry through sweat pores would be insignificant due to the small fraction of the skin surface occupied by such openings. Although a similar argument pertained to follicular penetration, it was acknowledged that under some conditions follicular penetration could be significant. The follicular route has some advantages for drug delivery in that it can accommodate nanoparticles and microparticles with potential slow drug release (Knorr et al. 2009; Lademann et al. 2011). A major question was whether interfollicular stratum corneum was amenable to transcellular penetration, paracellular penetration, or some combination of the two. This remained a point of contention until 1980, when Nemanic and Elias (1980) visualized the intercellular pathway followed by N-butanol diffusing across stratum corneum by in situ precipitation combined with transmission electron microscopy. The penetration pathway for this molecule was exclusively via the paracellular route. Squier and Lesch (1988) subsequently demonstrated an exclusively paracellular route for both polar and nonpolar small molecules using microaudioradiography.
So for passive diffusion of drugs across the stratum corneum, the paracellular route is considered the predominant pathway. Chemical permeability enhancers are thought to act primarily by fluidizing the intercellular lamellae of the stratum corneum, thereby reducing diffusional resistance (Thong et al. 2007; Ahad et al. 2009). Some physical means of enhancing drug delivery may also alter the intercellular lamellae; however some physical enhancement methods do alter pathways. For example, microneedle arrays create direct channels across the stratum corneum (Coulman et al. 2006). Iontophoresis may effectively deliver drugs through sweat ducts (Dixit et al. 2007). Sonophoresis may both alter the physical state of the intercellular lamellae and create transcellular pathways (Rao and Nanda 2009). Electroporation increases the permeability of the stratum corneum by opening or creating aqueous channels, through which relatively large water soluble molecules can pass (Singh et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree