Age
Chronically sun exposed areas of sun-damaged skin
Number of lentigenes
Number of AKs
History of previous keratinocyte carcinomas
Genetic conditions associated with LM include xeroderma pigmentosum, oculocutaneous albinism, Werner syndrome, and porphyria cutanea tarda [24–29]. These pigmentary or photosensitivity disorders increase the damage produced by ultraviolet radiation, perhaps leading to a quicker accumulation of DNA damage after cumulative sun exposure.
Progression of LM to LMM
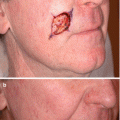
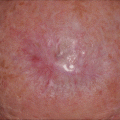
LM is a slow-growing lesion that often is diagnosed years after initial presentation. At first, these lesions can be easily misdiagnosed as benign solar lentigines. Occasionally, central regression is seen with extension of the peripheral margin, indicating continued growth and evolution of the lesion [30, 31]. Overall, the lifetime risk of progression from LM to LMM ranges upwards of 5–20 %, and may be increasing with time (Table 2.2) [32–36]. A retrospective epidemiologic study using data from the 1970s estimated risk of progression to be approximately 5 % [36]. Comparatively, studies using databases from single or dual institutions have estimated a risk of progression of up to 20 % [32–35]. Risk factors for transformation from LM to LMM is unclear, although larger size may be associated with diagnosis of LMM [32]. Timeframe for transformation to LMM is uncertain. In some patients, LM can be present for decades without progression to LMM. LM has no associated mortality, whereas prognosis of LMM is similar to other types of invasive melanoma [11].
Table 2.2
Lentigo maligna: risk of progression vs. incidence of unsuspected invasion
Author | Database | ||
|---|---|---|---|
Risk of progression from LM to LMM | Weinstock and Sober [36] | Health and nutritional examination survey from 1971–1974; Melanoma clinical cooperative group registry 1972–1977 | 5 %, age 45 2 %, age 65 |
Incidence of unsuspected invasion | Penneys [35] | Institutional pathology database, 1980–1987 | 15 % |
Somach et al. [34] | Institutional pathology database, 1992–1995 | 20 % | |
Hazan et al. [33] | Institutional surgical database, 2000–2006 | 16 % | |
Bousbous et al. [32] | Institutional surgical database, 1997–2008 | 10 % |
Role of Genetics
In the past decade, several genes have been associated with development of invasive melanoma, with mutations in BRAF commonly occurring early in the development of melanoma [37]. Discovery of these mutations has been especially groundbreaking due to the array of directed therapies now available for BRAF mutated tumors [38]. BRAF mutations were found in >50 % of LMM in one study which examined 13 LMM [39]. This frequency of mutation was similar to other subtypes of melanoma, including superficial spreading and nodular melanomas [39]. In 6 cases of LMM that lacked BRAF mutations, 1 case (17 %) was found to harbor an NRAS mutation [39]. In a meta-analysis of 36 studies involving BRAF mutations and 31 studies involving NRAS mutations, BRAF mutations were most commonly found in superficial spreading melanoma. More specifically, BRAF mutations were found in 49 % of superficial spreading melanomas compared to 22 % of LMM. This mutation was found to be significantly associated with superficial spreading melanomas. NRAS mutations were found in 17 % of superficial spreading melanomas and 14 % of LMM and was not significantly associated with either superficial spreading melanoma or LMM [40].
While BRAF is one of the most common mutations in melanomas, it is worth noting that several studies with larger sample sizes have shown that the majority of LM lesions tend not to exhibit any known mutation. Mutated specimens of LM demonstrated BRAF abnormalities ranged from 0–54 % of cases examined [39, 41–50]. A major limitation of these studies is the limited sample sizes, with the majority of studies examining <10 lesions. In LM lesions with a confirmed BRAF mutation, the BRAF V600K mutation was more prevalent than the BRAF V600E mutation, and was found in 16 % and 5 % of lesions, respectively [41].
Future Direction
Current research is under way to better delineate the epidemiologic risk factors and characteristics that help to distinguish LM from MIS. Beyond clinical factors, pathologic features unique to LM may also help to further clarify and separate this diagnosis from MIS. Genetic factors may also be helpful in the future to assist with differentiating these two lesions.
Conclusion
Our understanding of the epidemiology of LM continues to evolve. Risk factors for LM compared to non-LM melanoma are well studied, with several risk factors differing between the two conditions. However, it remains unclear why some lesions progress from LM to LMM whereas others stay indolent for decades as LM. The current era of genetics research in melanoma brings renewed hope for elucidating the underlying mechanism for the development of LM.
References
1.
2.
3.
4.
5.
6.
Clark Jr WH, Mihm Jr MC. Lentigo maligna and lentigo-maligna melanoma. Am J Pathol. 1969;55:39–67.PubMedPubMedCentral
7.
Golger A, Young DS, Ghazarian D, Neligan PC. Epidemiological features and prognostic factors of cutaneous head and neck melanoma: a population-based study. Arch Otolaryngol. 2007;133:442–7.CrossRef
8.
Cox NH, Aitchison TC, Sirel JM, MacKie RM. Comparison between lentigo maligna melanoma and other histogenetic types of malignant melanoma of the head and neck. Scottish Melanoma Group. Br J Cancer. 1996;73:940–4.CrossRefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree