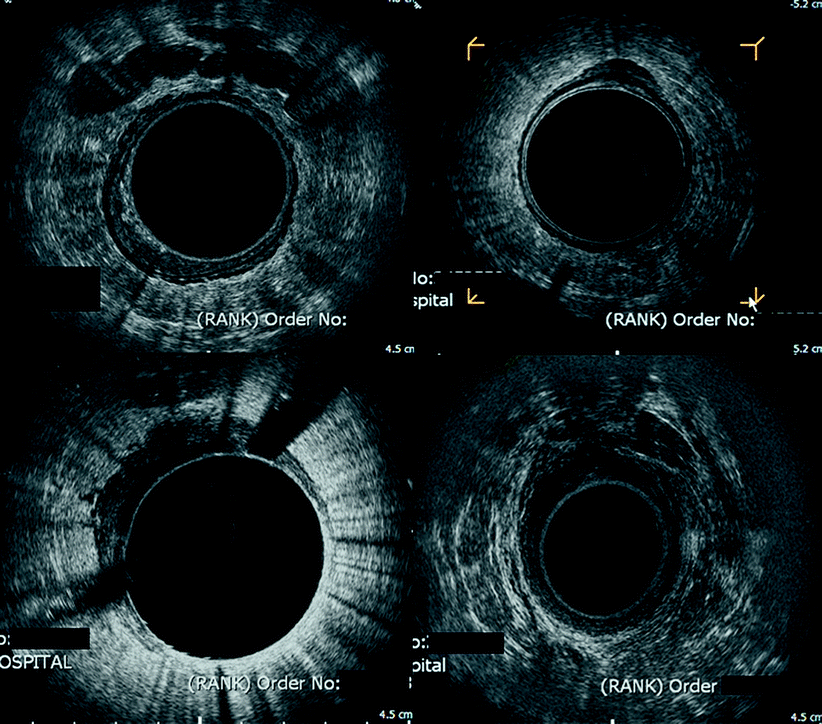
Fig. 3.1
Normal with prostate anteriorly. Normal endorectal sonography demonstrating the five layers as described in the text; the prostate gland can be seen anteriorly. In certain areas, seven layers can be seen, which reflects the interface between circular and longitudinal muscles of the rectum
First hyperechoic layer – interface between the water/balloon and the mucosal surface
Second hypoechoic layer – combined image produced by the mucosa and muscularis mucosae
Third hyperechoic layer – submucosa
Fourth hypoechoic layer – muscularis propria
Fifth hyperechoic layer – interface between the muscularis propria and perirectal fat or serosa, if present
ERUS also can identify the extrinsic anatomy of the uterus, vagina, prostate, and seminal vesicles and evaluate whether congenital fascial planes between the rectum and these structures are intact.
Three-Dimensional Ultrasound
During the last decade there has been increasing interest in the use of 3D ERUS, principally arising from the limitations of viewing a 3D structure with a two-dimensional (2D) image, as has been the case with traditional ERUS. Using the example of a rectal tumor, because only discrete 2D images can be assessed at any given time, no direct information concerning the longitudinal extent of the tumor and its spatial relationships is available. Consequently, a series of transverse images must be integrated by the observer to produce a mental image of the real anatomy [5].
Three-dimensional images are constructed from the synthesis of a high number of parallel, transaxial, 2D images [6]. This is only possible with suitable ultrasound apparatus and computer technology integrated with 3D software [7]. The resolution of 2D images is measured in pixels (each pixel having an x and y plane). In 3D ultrasound the pixel is transformed into a small 3D picture element called a voxel. The depth of the voxel is critical to the resolution of the 3D image: High-resolution 3D ultrasound typically acquires four to five transaxial images per millimeter acquisition of length in the z plane [7]. The images then are rendered using one of three basic techniques [6, 7]:
1.
Surface-based viewing technique: An operator or algorithm identifies the boundaries of the structures to create a wire-frame representation. This technique fails when a strong surface cannot be found, such as in the subtly layered structures of the anal canal.
2.
Multiplane-viewing technique: Three perpendicular planes (axial, transverse, and longitudinal) are displayed simultaneously and can be moved and rotated by the operator to visualize the lesion at different angles.
3.
Volume-render modes: The 3D image is projected onto a 2D plane by casting rays through the 3D image. The voxel values intersected by each ray can be multiplied by various factors and summated to produce different effects with variations of thickness, filtration, luminance, and opacity.
In the last decade the technique and efficacy of 3D ERUS have been evaluated in a number of disease states that traditionally have been the domain of conventional 2D ERUS, including the staging of rectal cancer [8, 9], identification of recurrent rectal cancer [10], perianal fistulous disease [11], and sphincter assessment in the investigation of incontinence [12]. Published data confirm that 3D imaging is feasible and may have some advantages over 2D techniques. However, the real advantages remain unclear [13].
Endorectal Ultrasound and Rectal Cancer
Historically, surgery for rectal cancer was performed as expediently as possible by blunt blind dissection that yielded poor oncological results with high rates of local failure. Over the last 30 years, changes in surgical technique, use of preoperative neoadjuvant therapy, and improvements in radiological staging have dramatically changed our approach to the treatment of rectal cancer. Surgically, total mesorectal excision (TME) with or without sphincter preservation for advanced lesions and transanal endoscopic microsurgery (TEMS) for selected early lesions are the norm. Neoadjuvant treatment has become routine for locally advanced tumors. Preoperative staging with both ERUS and magnetic resonance imaging (MRI) also have become the norm and have dramatically improved local staging accuracy. Consequently, patients diagnosed with rectal cancer today receive a tailored, evidence-based approach to both staging and treatment of their disease. When tailoring such an approach, some key questions must be addressed: Is the disease confined to the mucosa and submucosa? If so, can the disease be successfully managed without recourse to TME surgery? If the tumor has invaded the muscularis propria, are there any indications that the patient should be offered preoperative neoadjuvant treatment before surgical TME? Is it necessary that they undergo multivisceral resection? After neoadjuvant treatment, has the tumor responded to an extent that the surgical strategy can be modified or avoided all together? Both MRI and ERUS have established roles in answering each of these questions and will be discussed with a particular emphasis on ERUS.
With ERUS, rectal tumors have a hyopechoic appearance, and as the tumor invades deeper through the rectal wall, the normal sonographic anatomy is disrupted. By comparing the changes caused by a tumor with the normal sonogram the depth of tumor and, hence, an ultrasound T stage (denoted with the “u” prefix) [14] can be assigned to the tumor (Table 3.1) [15]. Examples of T1-4 rectal cancers are shown in Fig. 3.2.
TNM T stage | Histopathology | Ultrasonographic features |
|---|---|---|
Tx | Primary lesion cannot be assessed | Tumor depth not determined |
T0 | No primary tumor identified | No tumor seen |
Tis | Carcinoma in situ (limited to mucosa) | First hypoechoic layer is expanded but second hyperechoic layer is intact |
T1 | Tumor invades submucosa but does not involve muscularis mucosa | No disruption of the bright middle hyperechoic layer |
T2 | Tumor invades muscularis propria | Tumor confined by the hypoechoic layer of the muscularis propria with no disruption of the bright interface between it and surrounding fat |
T3 | Tumor invades perirectal fat/serosa | Outer hyperechoic layer disrupted, with the tumor edge usually irregular and has saw-tooth projections |
T4 | Tumor invades into neighboring organs/peritoneal cavity | Tumor extends into neighboring organs |

Fig. 3.2
Composite image of T1–4 rectal cancers. Endorectal sonography images demonstrating (top left) T1 rectal cancer with seminal vesicles seen anteriorly; (top right) T2 rectal cancer with invasion into the muscularis propria and only a small defect in the submucosa; (bottom left) T3 tumor with disruption of the outer hyperechoic, demonstrating invasion through the muscularis propria into perirectal fat; and (bottom right) T4 tumor in a male patient with prostatic invasion anteriorly, evidenced by disruption of Denovillier’s fascia
ERUS in Early Rectal Cancer
Patients with early mucosal disease without lymphatic involvement may be considered for endo-anal excision or TEMS. In this situation, multivariate analysis of 16 years of scientific literature has found ERUS to have a sensitivity of 94 % and a specificity of 86 % in determining invasion of the muscularis propria [16]. Comparing ERUS with MRI, the same meta-analysis found that the sensitivity of ERUS is equivalent to that of MRI but the specificity of ERUS is superior (86 % versus 69 %) [16]. In the context of this book, which focuses on reoperative pelvic and reconstructive perineal surgery, the role of ERUS in early rectal cancer will not be discussed further.
ERUS in Advanced Rectal Cancer
The majority of patients afflicted with rectal cancer present with disease that has penetrated into or beyond the muscularis propria (>T2 disease). These patients are thought to benefit from neoadjuvant radiotherapy or chemoradiotherapy (CRT). The aim of this treatment strategy is to downsize and downstage the more advanced primary tumor to improve local disease control. In addition, some authors believe that neoadjuvant therapy may allow modification of the subsequent surgical strategy, where some tumors may become suitable for local excision rather than formal TME/abdominoperineal excision [17–19] and where some tumors previously deemed surgically unresectable may become surgically resectable [20, 21]. Furthermore, some low rectal tumors may be downsized to such an extent that surgery to preserve the sphincter becomes feasible [22]. Up to 25 % of patients may achieve a complete response to CRT, which has led some authors to advocate a watch-and-wait policy rather than immediate surgical resection [23].
The Role of ERUS in the Identification of Locally Advanced Disease and Contiguous Organ Involvement (T4)
The distinction between <T3 and ≥T3 disease carries important treatment and prognostic information because there is decreased survival of patients with node-negative T3 and T4 disease compared with node-positive T1 or T2 disease [24]. Patients with ≥T3 disease are, therefore, those who typically are offered neoadjuvant therapy. Identification of extension of the primary tumor into adjacent viscera, typically the bladder, vagina, prostate, or seminal vesicles, is also vitally important during preoperative assessment so that en bloc multivisceral resection can be considered, which may improve the patient’s chance of cure [25, 26].
The roles of both ERUS and MRI in distinguishing between perirectal tissue invasion (T3) and adjacent organ involvement have been widely evaluated in the literature. The results of the 16-year meta-analysis of the literature comparing the modalities are presented in Table 3.2 [16]. This analysis demonstrated that ERUS had a greater sensitivity for identifying perirectal tissue invasion than MRI, but the modalities otherwise had similar accuracy, with both achieving a high specificity for adjacent organ involvement.
Table 3.2
Summary estimates of sensitivity and specificity for ERUS and magnetic resonance imaging (MRI) in the staging of rectal cancer [16]
Stage | Imaging modality | Sensitivity, % (95 % confidence interval) | Specificity, % (95 % confidence interval) |
|---|---|---|---|
Perirectal tissue invasion | ERUS | 90 % (88–92) | 75 % (69–81) |
MRI | 82 % (74–87)a | 76 % (65–84) | |
Adjacent organ invasion | ERUS | 70 % (62–77) | 97 % (96–98) |
MRI | 74 % (64–79) | 96 % (95–97) | |
Lymph node involvement | ERUS | 67 % (60–73) | 78 % (71–84) |
MRI | 66 % (54–76) | 76 % (59–87) |
ERUS and Nodal Involvement
The presence of lymphatic metastases is one of the strongest determinants of survival of and local failure of disease control in patients with rectal cancer. Consequently, patients who are identified as likely to have lymphatic involvement on the basis of preoperative imaging can be offered preoperative CRT in the hope of sterilizing the nodal disease before surgical resection. The success of ERUS in staging local invasion naturally led to its use to try and identify lymph node metastases. Although “normal” perirectal lymph nodes are not usually seen sonographically, “abnormal” malignant nodes often can be identified (Fig. 3.3) [27]. What sonographic features make malignant involvement more likely? Generally, metastatic nodes appear larger (>3 mm), hypoechoic, nonhomogenous, and more circular in shape, with well-defined borders. These features serve to distinguish them from inflammatory nodes, which tend to be more hyperechoic and oval in shape, with indistinct borders. Even with the knowledge of these discriminating factors, the accuracy of preoperative staging of lymphatic involvement with ERUS or MRI can be limited by the vague nature of some of the discriminatory characteristics. The size of the node also can be particularly unreliable for predicting malignant involvement [28] because small nodes can harbor small foci of malignant disease, whereas large nodes can merely be inflammatory. The previously quoted 16-year meta-analysis found that ERUS had a sensitivity of 67 % and a specificity of 78 % for lymphatic involvement, whereas MRI had a sensitivity of 66 % and a specificity of 76 % (see Table 3.2) [16]. ERUS and MRI therefore have limitations when trying to identify lymphatic involvement, but at present these modalities represent the best available techniques. The early hope that that positron emission tomography scanning may be useful in this regard has dissipated, as it has emerged that positron emission tomography/computed tomography (CT) lacks the ability to discriminate between the Flurodeoxyglucose (FDG)–avid primary tumor and positive nodes in close proximity to the tumor [29].
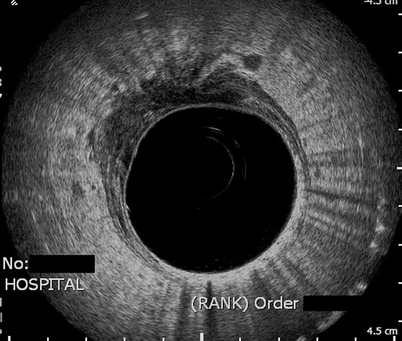

Fig. 3.3
T4 rectal cancer with perirectal enlarged lymph nodes. T4 tumor with invasion into the prostate anteriorly. Enlarged perirectal nodes are seen anteriorly and laterally at 9 o’clock
Pitfalls with ERUS
Although ERUS is clearly a useful staging modality for both early and locally advanced rectal cancer, there are some limitations that should be elaborated. The excellent results achieved in some institutions have not been mirrored in others, with reported overall accuracies of ERUS varying from 54 to 92 % [14, 30]. The reasons for this discrepancy may include the operator-dependent nature of the technique [31], which has a recognized learning curve, particularly for nodal assessment [32, 33]. Publication bias of more accurate results may also have give an artificially high estimate of the accuracy of ERUS [34]. When there is staging inaccuracy, it is usual that the patient is overstaged rather than understaged [31, 33], which may reflect caution on behalf of the sonographer, who fears the consequences of understaging a patient because of the potentially disastrous sequelae of such a diagnostic error. In addition, traditional 2D ERUS often is unable to define the TME plane; consequently, it has a limited role in decision making about potential circumferential resection margin (CRM) involvement and neoadjuvant therapy, where MRI is considered to be a much better modality for CRM evaluation. Recent work suggests that 3D ERUS may have a role in the evaluation of the CRM preoperatively [35], but at present there are limited data to routinely recommend this technique. Similarly, not all perirectal and inferior mesenteric artery lymph nodes are within reach of the sonographic image, and, again, MRI may have advantages over ERUS in identifying these nodes. ERUS also can be inaccurate in patients whose anatomy has been disrupted by factors such as tissue biopsy before ultrasound that has resulted in hematoma formation or after a polypectomy that has revealed a focus of malignancy that needs subsequent formal staging.
Neoadjuvant therapy can induce quite marked changes in the normal sonographic anatomy, which can make it difficult to differentiate between tissue reaction that is usual after CRT and ongoing malignant disease. The role of and difficulties with ERUS after CRT are explored further later.
ERUS After Chemoradiotherapy for Rectal Cancer
Neoadjuvant CRT is increasingly being used for the treatment of rectal cancer. As outlined previously, some authors feel that downstaging the tumor in this situation may negate the need for surgery in selected patients [23] or may allow for local excision rather than TME [17–19]. One of the challenges in these scenarios is to identify correctly those patients who can safely be managed in this way. CRT induces inflammation and subsequent fibrosis in the irradiated tissue, where these changes pose a significant problem for the clinician trying to accurately restage a patient after treatment, particularly if a watch-and-wait policy is contemplated, because these areas can harbor small foci of malignancy that may be difficult to identify but would mandate surgical intervention. Attempts to identify these foci or stage more obvious persisting pathology rely on a combination of clinical examination and imaging modalities such as ERUS.
Several authors have reported that the accuracy of local staging with any modality (ERUS, MRI, or CT) after CRT is dramatically reduced because of the difficulty of differentiating inflammation and scar from viable malignant tissue. Maretto and colleagues [36] studied ERUS, MRI, and CT imaging after CRT in 46 patients with mid/lower rectal tumors, and they found ERUS had an accuracy of predicting T stage of 64 % and of N stage of 61 %, which was similar to MRI and CT. Huh and colleagues [37] looked at the accuracy of ERUS (60 patients) and CT (80 patients) after CRT and found an accuracy of 38 % for T stage with ERUS against 46 % for CT and an accuracy of 73 % in ERUS and 70 % in CT for nodal staging. Interestingly, in this study none of the 11 patients who experienced a complete response were identified as such by either modality. In another study by Radovanovic and colleagues [38], 44 patients were evaluated with ERUS after CRT. This group reported an ERUS accuracy of 75 % for T staging and 68 % for N staging. Five patients in this series had a complete response, only one of whom was identified by ERUS preoperatively. Pomerri and colleagues [39] evaluated ERUS, MRI, and CT after CRT in 90 consecutive patients and found that all modalities had poor accuracy. T stage accuracy was as follows: 27 % by ERUS, 34 % by MRI, and 37 % by CT. The accuracies with N staging were 65, 68, and 68 % by ERUS, MRI, and CT, respectively. This study did, however, demonstrate that mural staging was much improved if the T stages were grouped as ≤T3 and T4; when grouped in this way, discrimination with ERUS was particularly accurate, with a sensitivity of 92 % and a specificity of 95 %. However, it must be recognized that there were only seven patients with T4 disease in this study.
Therefore, it can be seen that the major clinical problem with tailoring surgery to examination and imaging results after treatment is that there is no means of predicting with certainty which patients have had a total or near total response until the tumor is resected and examined histologically. Hence it is the authors’ practice to base all surgery after treatment on imaging results before rather than after CRT.
The Detection of Local Recurrence
Despite apparently successful primary radical surgery, local recurrence occurs in up to 12 % of cases of rectal cancer [40]. Local recurrence is more common in tumors that are located in the lower third of the rectum, those that are large and locally invasive, and those with lymphatic metastases. When ultrasound scanning after surgery it must be remembered that the anatomy of the pelvis may have been altered by the operation and image interpretation immediately after surgery may be hampered by early normal post-operative changes. Therefore, scanning should ideally be deferred until 3 months after surgery. The common so-called anastomotic recurrence more likely results from local recurrence within the pelvis, which breaks through and presents at the anastomosis.
Is there any evidence that ERUS – which is so accurate in the preoperative staging of rectal cancer – is of any use in the assessment and diagnosis of local tumor recurrence? Because it is relatively inexpensive and portable and the examination is of short duration, it could be included in routine follow-up along with clinical examination and sigmoidoscopy. Female patients treated by restorative resection or abdominoperineal excision also can be scanned transvaginally. Assessment of the neorectum is, in essence, no different than preoperative examination in that the five layers still can be clearly identified. The presence of a stapled colorectal anastomosis does not affect the interpretation of the images, where staples are seen as small bright echoes without any attendant acoustic shadowing. The ultrasonic anatomy of the pelvis may alter after surgery; scanning is ideally performed approximately 3 months after treatment, and care is required during interpretation.
Established locally recurrent cancer, detectable by digital and sigmoidoscopic examination, has an endosonographic appearance identical to that of primary rectal cancer because it is echo-poor in nature. The extent of invasion can be assessed as with primary tumors because, again, there is disruption of the recognizable ultrasonic layers. Extrarectal locally recurrent tumors can be detected at an early stage. Here, tumors appear as a circumscribed echo-poor area within the para-anastomotic tissues, although the presence of tumors cannot always be diagnosed easily using only ERUS technology.
In these situations, one of two policies can be adopted, namely (Fig. 3.4):
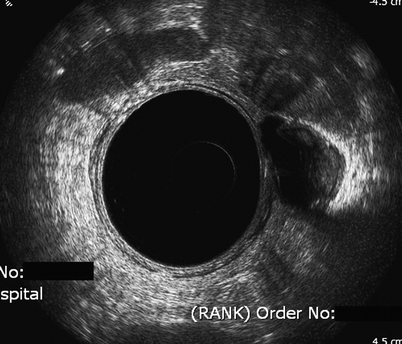

Fig. 3.4
Possible recurrence. Endorectal sonography scan of a male patient 6 months after anterior resection. A concerning mixed echogenic shadow is seen at the 3 o’clock position. Options here include repeat scan in 3 months or guided transperineal biopsy
1.
A repeat ultrasound scan can be performed after a suitable period of a month or 6 weeks. An increase in size usually will indicate recurrent malignancy.
2.
A percutaneous transperineal biopsy can be performed using the endoprobe as a needle guide.
The effectiveness of ERUS in the follow-up of patients has been reported from a few centers. Twenty-two recurrences were detected by Hildebrandt et al. [41] using ERUS, but only six of these were noted with ultrasound alone. Three cases also had an elevated carcinoembryonic antigen level, whereas ten cases had digital or endoscopic signs with an elevated serum carcinoembryonic antigen. Eight local recurrences were detected by Romano and colleagues [42] in their follow-up of 42 patients. Two ultrasonically false-positive cases of fibrosis were confirmed by percutaneous ultrasound-guided biopsy. Beynon et al. [43] imaged 22 recurrences in 85 patients, of which only three were detected solely by ERUS. All other recurrences in this study could be palpated digitally or were obvious during sigmoidoscopic examination.
In another study from Italy, 120 patients were followed up by Mascagni et al. [44]. Seventeen recurrences were detected in this analysis, of which six were asymptomatic. Twelve recurrences were detected by endorectal ultrasound, whereas five were found using endovaginal sonography, resulting in an accuracy of 97 %, with a sensitivity and a specificity of 94 and 98 %, respectively. In six of their patients, recurrences could be detected by either digital examination or by simple endoscopy. Morken and colleagues [45] reported their experience with 525 patients followed up with ERUS after local or radical rectal resection; in this series, any lesion suggestive of local recurrence was evaluated with an ERUS-guided biopsy. Thirty-nine patients were documented to have local failure during follow-up, of which five (13 %) were detectable only with ERUS. Eighty-two percent of patients had a diagnostic biopsy at the initial ERUS. Doornebosch and colleagues [46] reported a series of T1 rectal tumors treated by TEMS; of 18 recurrences, 6 were detectable only on ERUS examination.
Thus, small, extrarectal recurrences can be detected using ERUS before there is any evidence of luminal recurrence, which may secondarily benefit from guided biopsy. The routine use of ERUS after the surgical treatment of rectal cancer also permits a detailed examination of the pelvis, which was not previously possible without the use of more expensive techniques such as CT scanning or MRI. In this context, ERUS has a potential role after both local excision with the TEMS technique and TME. When used routinely from 3 months postoperatively, ERUS would possibly allow the detection of early recurrence in a larger number of patients at a stage when there may be an attempt at cure from reoperative surgery.
Overview
Much of the published literature about techniques used in local staging of rectal cancer and postoperative follow-up has attempted to answer the question, Is ERUS or MRI the more accurate modality? In reality, both techniques have their advantages (Table 3.3), which individually may yield useful information that can be used synergistically to optimize decision making for the individual patient (Fig. 3.5). It is, therefore, the authors’ opinion that both techniques should be used routinely in all cases of rectal cancer.
Table 3.3
Comparison of the merits and disadvantages of endorectal sonography (ERUS) and magnetic resonance imaging (MRI) in the management of rectal cancer
ERUS | MRI | |
|---|---|---|
Identification of early mucosal disease | Superior |









