Fig. 16.1
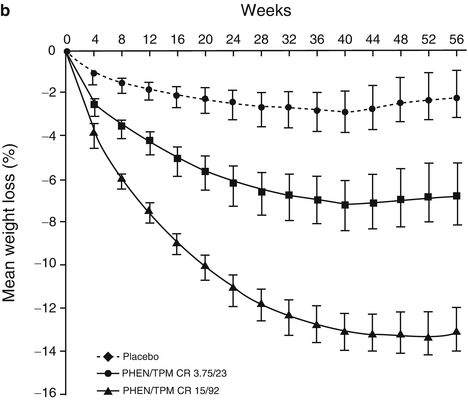
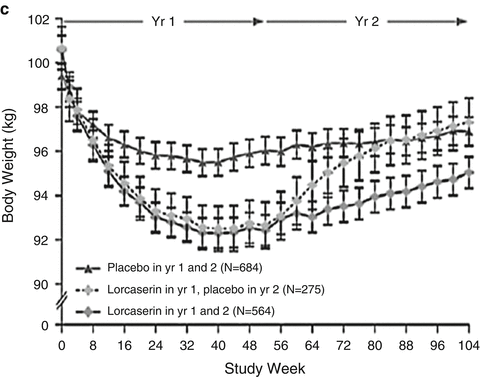
Weight loss effects of the FDA approved weight loss drugs. (a) The effect of Phentermine 30 mg once daily on body weight during continuous or intermittent administration (1 week on, 1 week off) for 36 weeks during a randomized controlled clinical trial. Both treatment groups lost significantly more weight than the placebo group, but there was no significant difference between the two treatment groups. Data presented as mean. Adapted from Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. British Medical Journal. Feb 10 1968;1(5588):352–354 with permission from BMJ Publishing Group Ltd. (b) The effect of Phentermine/Topiramate 3.75 mg/92 mg or 15 mg/92 mg once daily on body weight in a 56-week randomized controlled clinical trial. Both treatment groups resulted in significantly moreFig. 16.1 (continued) weight loss compared with placebo group, with the bigger dose reaching significantly more weight loss. Data shown from the intention-to-treat population and represented as mean ± 95%CI. Abbreviations: Phen/TPM (CR 3.75/23), Phentermine (3.75 mg/Topiramate 23 mg). Phen/TPM (CR 15/92) Phentermine (15 mg)/Topiramate (92 mg). Adapted from Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity. Feb 2012;20(2):330–342 with permission from John Wiley and Sons. (c) The effect of Lorcaserin 10 mg twice daily for 52 weeks followed by 52 weeks of placebo or 104 weeks of Lorcaserin alone in a randomized controlled clinical trial. During the first 52 weeks, the treatment group lost significantly more weight than placebo group. At 104 weeks, all groups gained weight compared to 52 weeks, although the subjects maintained on Lorcaserin for 104 weeks had significantly more weight loss compared to baseline and compared to patients on placebo for 104 weeks or Lorcaserin for 52 weeks switched to placebo for 52 weeks. Data presented as mean ± standard errors. Adapted from Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England Journal of Medicine. Jul 15 2010;363(3):245–256 with permission from Massachusetts Medical Society
Indications
Phentermine is indicated as a short-term adjunct for weight loss management with exercise and caloric restriction in patients with body mass index (BMI) ≥ 30 kg/m2 or 27 kg/m2 with at least one weight related comorbidity (e.g. controlled hypertension, diabetes, and hyperlipidemia). Laws vary state to state regarding acceptable duration and extent of treatment and local guidance should be sought before prescribing.
Mechanism of Action
Phentermine is a sympathomimetic amine with anorectic effects, probably due to the stimulatory effects on hypothalamus to release norepinephrine [1].
Efficacy
Phentermine is currently the most popular drug prescribed and used for weight loss [2]. This is probably due to the low cost, physician experience, efficacy, and a paucity of medical alternatives prior to 2013. The efficacy of Phentermine for weight loss has been evaluated in a meta-analysis of nine randomized controlled trials published between 1975 and 1999 [3] that used Phentermine short term. These studies involved mostly women (more than 80 %) treated with Phentermine for 2–24 weeks in addition to lifestyle modification (more than 80 % of the participants). Overall there was 3.6 kg (CI, 0.6–6.0 kg) additional weight loss compared to placebo in the subjects treated with Phentermine 15–30 mg daily [3]. Due to concerns around tachyphylaxis and the lack of data on long-term safety, Phentermine 30 mg daily was administered either continuously, or intermittent (4 weeks on, 4 weeks off) in a double-blinded placebo-controlled study with healthy overweight and obese subjects treated for 36 weeks in addition to a calorie restriction diet [4]. Munro et al. showed that there was no significant difference in amount of weight loss with intermittent Phentermine vs. continuous Phentermine treatment (13 kg vs. 12.2 kg, respectively), but there was a significant difference compared to placebo group weight loss (4.8 kg) [4] (Fig. 16.1a). A recent retrospective study of patients treated with Phentermine for 12 weeks to 12 years in addition to calorie restricted diet showed that the patients on Phentermine lost significantly more weight compared to the no Phentermine group [5]. Importantly, some patients in the Phentermine group continued to maintain more than 10 % weight loss for as long as 8 years [3]. The latter study is limited by the small number of patients in the long-term follow-up period. As part of the clinical development of Phentermine/Topiramate, Phentermine was studied as a monotherapy [5]. On a background of a lifestyle intervention (weight loss of 4.1 + 7.5 kg), Phentermine produced greater weight loss (10.2 + 6.9 kg) over 156 weeks [5].
Side Effects/Tolerability
Like all sympathomimetic amines, Phentermine has been reported to have cardiovascular side effects (palpitations, tachycardia, elevated blood pressure, ischemic events); central nervous system side effects (overstimulation, restlessness, dizziness, insomnia, euphoria, dysphoria, tremor, headache, psychosis); gastrointestinal side effects (dryness of mouth, unpleasant taste, diarrhea, constipation); allergic side effects such as urticaria; and endocrine side effects including impotence and changes in libido. Valvular heart disease and primary pulmonary hypertension have been reported in only rare cases of patients taking Phentermine alone [1] (Table 16.1). These cases probably represent the background of cardiac valvulopathy in the general population as several studies have not found evidence for valvular heart disease or primary pulmonary hypertension in patients using Phentermine as monotherapy [3, 5].
Contraindications
Phentermine is contraindicated in patients with a history of cardiovascular disease (defined as uncontrolled hypertension, stroke, arrhythmia, coronary artery disease, heart failure), during or within 14 days of treatment with monoamine oxidase inhibitor, in hyperthyroidism, in glaucoma, in agitated states, with a history of drug abuse, in pregnancy, during nursing or with known idiosyncratic reactions to the sympathomimetic amines [1].
Drug Interaction
The concomitant use of Phentermine should be avoided with: monoamine oxidase inhibitors, alcohol, and adrenergic neuron blocking drugs [1].
Monitoring
Blood sugar should be monitored carefully in patients with diabetes, particularly during the initiation of therapy so that insulin and oral glucose lowering medications can be adjusted as needed to prevent hypoglycemia. In patients with tight glucose control, and depending on the antihyperglycemic agents used, down titration of those medications should be considered when initiating treatment with a weight loss drug. Blood pressure and heart rate should be monitored, especially in patients with hypertension [1].
Safety
Phentermine is pregnancy category X. Phentermine is not recommended in lactating mothers or children (less than 16 years old) due to potential side effects. Phentermine should be used with caution in elderly patients and patients with renal impairment due to potential of excessive accumulation. Because Phentermine is related to amphetamine (which has high abuse potential) Phentermine is a controlled substance and there is a warning of abuse potential in the drug insert [1]. However, Hendricks et al. [6, 7] showed that Phentermine does not induce psychological dependence and abrupt cessation does not induce Phentermine cravings. Symptoms experienced after abrupt cessation represent loss of therapeutic effects, not amphetamine-like withdrawal symptoms [6, 7].
Phentermine/Topiramate
Early studies on the use of Topiramate [8] for weight loss led to the logical next step combining Phentermine with Topiramate. The combination was approved by the FDA in July 2012. Topiramate has been used for the treatment of seizure disorder since 1996; weight loss was noted as a side effect.
Indications
Phentermine and Topiramate extended release (trade name Qsymia™) is indicated for chronic weight management in adults with a BMI ≥ 30 kg/m2 or 27 kg/m2 with at least one weight related comorbidity (for example: type 2 diabetes, hypertension, hyperlipidemia) [9]. Of note, Phentermine/Topiramate has not been studied in conjunction with other weight loss treatments and long-term safety, specifically regarding cardiovascular morbidity and mortality is not known at this time.
Mechanism of Action
The mechanism of action for Topiramate is unknown. However, the effect of Phentermine is likely mediated by catecholamine release that reduces appetite and decreases food intake. The effect of Topiramate may be due to increased activity of the neurotransmitter gamma-aminobutyrate, modulation of voltage-gated ion channels, inhibition of AMPA/kainite excitatory glutamate receptors, or inhibition of carbonic anhydrase, which decreases appetite and increases satiety [9].
Efficacy
Pivotal studies of Phentermine/Topiramate extended release included a broad range of patients both with and without weight related comorbid conditions. Specifically, the efficacy of Phentermine/Topiramate was studied in a randomized, double-blinded, placebo-controlled study in obese patients with BMI more than 35 kg/m2 without diabetes (EQUIP study) [10]. Subjects (N = 514) were 18–71 years old, mostly women (83 %) and mostly Caucasian (80 %) with baseline weight of 116 kg. Treatment consisted of two doses of Phentermine/Topiramate (3.75 mg/92 mg and 15 mg/92 mg) or placebo in addition to nutrition/lifestyle modification counseling and a 500 kcal/day decrease in caloric intake with a balanced diet for 1 year. Both treatment dosages resulted in increased weight loss as compared with placebo (3.5 and 9.4 kg). The percentage of subjects losing greater than or equal to 5 % weight at the two doses was 27.6 % and 49.4 % or 10 % weight was 11.4 % and 39.4 % in the 3.75 mg/25 mg and 15 mg/92 mg Phentermine/Topiramate groups respectively [10] (Fig. 16.1b).
Subsequently, Phentermine/Topiramate efficacy was studied in 994 obese (BMI ≥ 30 kg/m2) and overweight subjects (BMI ≥ 27 kg/m2) with two or more significant comorbidities including hypertension, dyslipidemia, diabetes, prediabetes, or abdominal obesity (CONQUER study) [11]. Treatment dosage was either 7.5 mg/92 mg or 15 mg/92 mg. Subjects were between 18 and 70 years old, mostly women (70 %) and mostly Caucasian (86 %) with a baseline weight of 103 kg. In both studies, a substantial fraction of subjects withdrew from the study: 40 % in the EQUIP study with only obese subjects and 31 % in the CONQUER study with obese or overweight subjects with one or more comorbidity. Both treatment dosages resulted in increased weight loss as compared with placebo (6.6 and 8.6 kg). The percentage of subjects losing more than 5 % weight was 41.3 % and 49.2 %, or 10 % weight (29.9 % and 40.3 %) in the 7.5 mg/46 mg and 15 mg/92 mg Phentermine/Topiramate groups respectively.
The effect on weight maintenance was studied in a non-randomized extension of the overweight and obese SEQUEL trial for 52 more weeks in 676 subjects [12]. Phentermine/Topiramate 7.5 mg/46 mg and 15 mg/92 mg were found to be effective in maintaining weight loss for up to 108 weeks compared to baseline vs. placebo, respectively (7.5, 8.7 %). Phentermine/Topiramate significantly decreased waist circumference [10, 11]. Heart rate, systolic and diastolic blood pressure, cholesterol, and fasting glucose significantly improved with the 15 mg/92 mg dosage in the EQUIP trial [10] and CONQUER trial [11] and with 7.5 mg/46 mg Phentermine/Topiramate in the overweight and obese CONQUER trial [11]. In patients with and without diabetes, HbA1C significantly improved with both doses used in the CONQUER trial [11]. The development of new cases of diabetes was also decreased suggesting utility in diabetes prevention and treatment. Please note that Phentermine/Topiramate is not approved for use in the treatment of diabetes per se but is approved for use to manage weight in patients with diabetes.
Side Effects/Tolerability
The most common side effects associated with Phentermine/Topiramate use are paresthesia, dizziness, dysgeusia, insomnia, constipation, and dry mouth. If used in the first trimester of pregnancy, there is a higher risk for fetal oral clefts from the Topiramate. If Phentermine/Topiramate is discontinued abruptly, seizures may occur [9]. There is a known potential for abuse and dependence due to the Phentermine component [9] (Table 16.1). See the note on the addiction potential of Phentermine, as described in the Phentermine section.
Contraindications
Phentermine/Topiramate should not be used in pregnant patients, patients diagnosed with glaucoma, hyperthyroidism, recent/unstable cardiac or cerebrovascular disease, patients taking monoamine oxidase inhibitors or within 14 days of treatment, patients with known hypersensitivity or idiosyncrasy to sympathomimetic amines [9]. Caution should be used in women of childbearing potential [9].
Drug Interactions
Phentermine/Topiramate interacts with: (1) oral contraceptives and may produce irregular vaginal bleeding without an increase in pregnancy risk (discontinuation of oral contraceptives is not indicated); (2) CNS depressants including alcohol by potentiating CNS depressant effects (instruct to avoid concomitant use of alcohol); (3) potassium sparing diuretics by potentiating hypokalemia (monitor potassium before and during treatment); (4) antiepileptic drugs may decrease Topiramate concentration, increase blood ammonia, or produce hypothermia [9].
Monitoring
The absence of pregnancy should be confirmed in women of childbearing age by pregnancy testing before initiating therapy and monthly during treatment, due to potential for teratogenic side effects. In all patients, heart rate, suicidal behavior and ideation, acute myopia, secondary angle closure glaucoma, mood and sleep disorder, cognitive impairment, electrolytes, and creatinine should be monitored. Glucose control and diabetic medication adjustments should be performed in patients with diabetes. See note above on initiation of weight loss drugs in patients with diabetes. In patients with renal or hepatic impairment, the maximum dose used should not exceed 7.5 mg/46 mg [9].
Safety
Phentermine/Topiramate is pregnancy category X and therefore contraindicated due to increase in fetal oral clefts. Safety and effectiveness have not been established and therefore use is not recommended in nursing mothers, labor, and pediatric/geriatric patients [9]. A cardiovascular safety trial is anticipated to establish long-term safety.
Lorcaserin Hydrochloride
Serotonergic agents have been known to be effective for weight loss for more than 40 years. In the 1990s Fenfluramine was used to treat obesity. Subsequently, the d-isomer of Fenfluramine (dexfenfluramine) was approved by the FDA in 2013 for weight loss. Practically, most of the Fenfluramine prescribed was combined with Phentermine off label and the phrase Fen/Phen was coined to describe this use. Unfortunately, cardiac complications, specifically, cardiac valvulopathy was discovered in patients taking this combination. Later, in vitro studies implicated Fenfluramine and dexfenfluramine, not Phentermine, as agonists of the 5-HT2B serotonin receptors expressed on the interstitial cardiac valve cells. Agonists of the 5-HT2B serotonin receptors stimulate the growth of the interstitial cells with subsequent valvular incompetence also known as valvulopathy. Valvulopathy is a condition that occurs with many serotonergic drugs and with serotonin producing carcinoid tumors that metastasize to the lungs (reviewed in [13]). At about the same time, preclinical data identified the 5-HT2C serotonin receptor in the hypothalamus as critical for the weight loss effects of serotonergic agents. This led to the search, for 5-HT2C specific serotonin receptor agonists, which has proven to be very difficult. Lorcaserin was designed to be a selective agonist for the 5-HT2C serotonin receptor to retain weight loss efficacy without causing cardiac valvulopathy.
Indication
Lorcaserin is indicated as an adjunct to diet and exercise for weight loss and maintenance in obese patients (BMI ≥ 30 kg/m2) or overweight subjects (BMI ≥ 27 kg/m2) with at least one weight related comorbidity (for example: glucose intolerance, type 2 diabetes, hypertension, hyperlipidemia, and sleep apnea). Importantly, the FDA approved prescribing information mandates that Lorcaserin should be discontinued if less than 5 % weight loss has been achieved following 12 weeks of treatment [14].
Mechanism of Action
Lorcaserin decreases weight by reducing food consumption [15]. These effects are mediated through the activation of the 5-HT2C serotonin receptor located throughout the central nervous system. Serotonin acts in the hypothalamus to release αMSH and decrease AgRP release, which modulate appetite by increasing satiety and decreasing hunger [14]. Lorcaserin is thought to activate these same hypothalamic appetite control systems [13].
Efficacy
The efficacy of Lorcaserin as a weight loss drug in conjunction with behavior modification was demonstrated in three pivotal phase 3 studies. The 2 year BLOOM study [16] and the 1 year BLOSSOM study [17] included obese (BMI ≥ 30 kg/m2) or overweight subjects (BMI ≥ 27 kg/m2) with at least one weight related comorbidity. The BLOOM-DM study included patients with type 2 diabetes [18] treated for 1 year.
Lorcaserin produced significant body weight loss as early as 2 weeks after starting the treatment compared to placebo, and resulted in weight loss of approximately 5.8 % vs. 2.5 % in the BLOOM [16] and BLOSSOM [17] studies, respectively. In patients with diabetes in the BLOOM-DM weight loss averaged 5 % vs. 1.5 % in placebo-treated patients [18]. As with most other weight loss drugs, Lorcaserin was more effective in nondiabetic patients. After 1 year of treatment, Lorcaserin 10 mg orally twice daily led to at least 5 % weight loss in twice as many subjects compared with placebo (47 % vs. 23 % in pooled BLOOM [16] and BLOSSOM [17]; and 37 % vs. 16 % in BLOOM-DM [18]). Lorcaserin led to more than 10 % weight loss in 22 % vs. 9 % of control subjects in the pooled BLOOM [16] and BLOSSOM [17]. In BLOOM-DM, 10 % weight loss was achieved in 16 % of Lorcaserin-treated patients vs. 4 % in placebo-treated patients [18]. In the BLOSSOM and BLOOM Lorcaserin 10 mg orally twice daily was superior to once daily dosing therefore the Lorcaserin is recommended as 10 mg twice daily. Interestingly, in BLOOM-DM, once daily was almost as efficacious as twice daily dosing [18].
The efficacy of Lorcaserin 10 mg orally twice daily as a weight maintenance drug was demonstrated in the BLOOM [16] phase 3 study. After 1 year patients randomized to the placebo group remained in the placebo group, while patients assigned to Lorcaserin were randomized at the end of 1 year to either placebo or continued on Lorcaserin. As shown in Fig. 16.1c the patients who were maintained on Lorcaserin for 2 years were better able to maintain weight loss.
Side Effects/Tolerability
Common side effects reported with Lorcaserin include: headache, dizziness, nausea, fatigue, dry mouth and hypoglycemia, back pain, cough, and fatigue in type 2 diabetic patients. These are self-limited and once resolved did not re-occur [14] (Table 16.1). Lorcaserin has less selectivity for the 5-HT2A serotonin receptor than the 5-HT2C receptor which may explain some of these side effects [13].
Serious adverse events were rare and occurred in a similar number of patients in treatment vs. placebo in the phase 3 trials. An echocardiographic safety monitoring program showed no evidence for an increase in the risk of clinically significant valvulopathy [19]. Lorcaserin did not prolong QTC interval and did not increase heart rate or blood pressure. Psychiatric effects were evaluated in the phase 3 program. These studies showed a low potential for abuse, no increase in depression, anxiety, suicidal ideation or other mood disorders, and no cognitive adverse effects. Serum prolactin levels were moderately elevated [14].
Contraindications
Lorcaserin should not be used in patients with severe renal impairment (defined as a creatinine clearance <30 mL/min) due to potential accumulation of Lorcaserin metabolites. Lorcaserin should not be used in pregnancy [14].
Drug Interaction
Lorcaserin is a weak to moderate inhibitor of CYP2D6; however, clinical studies demonstrate a weak interaction with other CYP2D6 substrates, therefore no recommendation for dose adjustment is made. Caution should be used when combining Lorcaserin with serotonergic drugs [14].
Safety
Lorcaserin is contraindicated in pregnancy (Category X). Lorcaserin should not be used during lactation, or lactation should be discontinued prior to starting Lorcaserin. Pediatric use has not been studied, and therefore is not recommended [14].
Off Label Use of Drugs for Weight Loss
Bupropion
Bupropion was approved by FDA for treatment of depression in 1985 and smoking cessation in 1997. An anorectic effect was reported from a series of clinical observations [20] and Bupropion was further studied for weight loss as monotherapy or in combination with other drugs.
Indications
Bupropion is approved for use in depression and smoking cessation [21] and is sometimes used off label for weight loss in obese patients due to the anorectic effect.
Mechanism of Action
The exact mechanism of action for Bupropion is unknown, but it is thought to act through the inhibition of the neuronal reuptake of norepinephrine and dopamine [21].
Efficacy
The first report of Bupropion use for weight loss in subjects without depression was a short (8 weeks) randomized double-blinded, placebo-controlled trial in 50 overweight and obese subjects [20]. Subjects were treated with either placebo or Bupropion 100 mg/day and gradually increased to 200 mg/day in addition to a low-calorie diet (1,600 kcal/day). In 8 weeks, the net weight loss in the Bupropion group was 4.4 kg from baseline. The study was continued for 16 more weeks in responders (subjects who lost more than 5 % of baseline weight in 8 weeks) and they lost an additional 12 kg, from which fat loss was 73 % [20].
A longer study of Bupropion was reported in 2002, where 327 subjects were enrolled in a multicenter double-blinded, placebo-controlled study with Bupropion SR 300 or 400 mg/day in addition to calorie restriction, meal replacements, and exercise [22]. Subjects weighed an average of 100 kg at baseline, and were mostly middle aged women. After 24 weeks there was a significant dose dependent net weight loss in the Bupropion 300 mg/day (2.2 %) vs. Bupropion 400 mg/day (5.1 %). In a 24-week extension, subjects taking Bupropion 300 mg/day lost a total of 7.5 % of initial body weight vs. 8.6 % in the Bupropion 400 mg/day arm [22].
In a double-blinded placebo-controlled randomized clinical trial of 419 patients with uncomplicated obesity, the effect of sustained-release Bupropion SR 400 mg/day monotherapy, immediate release Naltrexone monotherapy (48 mg/day), and the combination of both drugs was studied for 24 weeks [23]. In contrast to the study by Anderson, monotherapy with Bupropion sustained-release 400 mg/day led to a net weight loss of only 2 % as compared to placebo [23]. The effects of Naltrexone and the combination will be discussed in more detail below.
A meta-analysis on the effect of Bupropion in depressed patients [24] included 13 studies published from 1982 to 2006. Serretti et al. concluded that Bupropion produced a significant net weight change of −1.13 kg (95%CI −1.41 to −0.84, p < 0.0001) during acute treatment (4–12 weeks) and −1.87 kg (95%CI −2.37 to −1.37, p < 0.0001) during medium and long-term treatment (more than 4 months) [24].
Of all antidepressants with weight loss effects (imipramine, nortriptyline, citalopram, escitalopram, fluoxetine, paroxetine, sertraline, duloxetine, mirtazapine) Bupropion was the only one to demonstrate weight loss maintenance in long-term treatment [24]. Of note, most of these other agents act on the serotonin system [24].
Side Effects/Tolerability
Common side effects of Bupropion include: agitation, insomnia, anxiety, dry mouth, headache, dizziness. Serious side effects include suicidal ideation, psychiatric disorders, hallucination, tachycardia, and hypertension [21] (Table 16.1). There are no long-term cardiovascular outcome trials of Bupropion. Given a mechanism of action that is similar to sibutramine, the authors recommend caution in using this agent in patients with a history of or high risk for cardiovascular disease.
Contraindications
Bupropion is contraindicated in patients with seizure disorder, eating disorders, patients undergoing abrupt cessation of alcohol or sedatives or within 14 days of treatment with monoamine oxidase inhibitors [21].
Drug Interaction
Bupropion is metabolized in liver by the CYP2B6 isoenzyme, therefore may interact with other drugs that inhibit or induce CYP2B6 including: MAO inhibitors, amantadine, levodopa, tramadol, warfarin, clopidogrel, olanzapine, and systemic corticosteroids. Bupropion should be avoided with drugs that lower seizure threshold (like antipsychotics or other antidepressants) [21].
Safety
Bupropion is pregnancy category C, therefore should be used only if risks of discontinuation outweigh the benefits. It is possibly unsafe in lactation due to its secretion in human milk; therefore, Bupropion should be discontinued in these cases. Bupropion is not approved for use in children and should be used with caution in the elderly, due to probable impaired renal function [21].
Black Box Warnings
Bupropion has a black box warning of increasing suicidal ideation, especially in children and young adults; their close clinical monitoring is recommended especially in the beginning of therapy [21].
Topiramate
Topiramate was initially studied as an antidiabetic drug to inhibit gluconeogenesis. In the process of research an anticonvulsant effect was observed and subsequently Topiramate was FDA approved in 1996 for this indication. Later on, an effect on appetite was observed [25] and Topiramate was further tested for weight loss in mono or combination therapy.
Indications
Topiramate is approved for treatment of seizures and for migraine prophylaxis [26] and has been used off label for weight loss.
Mechanism of Action
The exact mechanism of action is unknown, but there is some evidence for voltage dependent sodium channel blockade, augmentation of gamma-aminobutyrate (GABA) activity, antagonizing the glutamate receptor, and inhibition of carbonic anhydrase [26].
Efficacy
The effects of Topiramate on weight was studied first in obese subjects or overweight subjects with BMI ≥ 27 kg/m2 with hyperlipidemia or/and hypertension [27]. This randomized double-blinded, placebo-controlled dose-ranging trial studied the effect of Topiramate 64, 96, 192, or 384 mg/daily for 6 months on weight in addition to low-calorie diet and exercise counseling/monitoring. The study enrolled 385 subjects, and at the end of 6 months there were 242 completers. Net placebo adjusted weight loss at the end of study was 2.1 kg, 2.9 kg, 4.4 kg, 5 kg in the 64 mg/day, 96 mg/day, 192 mg/day or 384 mg/daily, respectively. Interestingly, weight loss started at 4 weeks and continued to 6 months without reaching a plateau. The responder rate (those losing more than 5 % body weight) was significantly higher in Topiramate in the 64 mg/day (49 %, p = 0.03), 96 mg/day (59 %, p = 0.02), 192 mg/day (70 %, p = 0.001) or 384 mg/daily (61 %, p = 0.007) groups as compared to placebo (29 %) [27].
Subsequently, the effect of Topiramate monotherapy in doses of 96, 192, and 256 mg/day was studied over 60 weeks in conjunction to the “Pathway to change” lifestyle intervention [28]. Subjects were obese or overweight with a BMI ≥ 27 kg/m2 and had hyperlipidemia and/or hypertension. This randomized, placebo-controlled, double-blind study enrolled 1,289 subjects and 709 completed the 60-week trial. Topiramate induced a net weight loss compared to placebo of 5.9 kg (p < 0.001), 7.9 kg (p < 0.001), 8.4 kg (p < 0.001) in the 96 mg/day, 192 mg/day, and 256 mg/day, respectively. Among the Topiramate groups, weight loss in the 96 mg/day vs. 192 mg/day Topiramate was significantly different, but similar between 196 mg/day and 256 mg/day Topiramate. Responders (losing more than 5 % body weight) in the placebo and 96 mg/day, 192 mg/day, and 256 mg/day Topiramate groups were 18 %, 54 %, 61 %, and 67 % respectively. Weight loss was accompanied by a significant improvement in blood pressure, fasting glucose, 2 h glucose and insulin in all groups. Only the 96 and 256 mg/day Topiramate significantly improved LDL in this predominately normo-lipidemic population [28].
The effects of Topiramate on weight loss were summarized in a 2011 meta-analysis [29] which included 11 randomized, controlled trials published between 2003 and 2007. The analysis included 3,320 individuals treated with Topiramate for at least 16 weeks. Topiramate induced a 5.34 kg net weight loss (95%CI −6.12, −4.56) compared to placebo. This analysis also concluded that weight loss did not reach a plateau by 28 weeks [29].
Side Effects/Tolerability
Common side effects of Topiramate are flushing, loss of appetite, altered taste, confusion, impaired memory and psychomotor performance, and paresthesias. Serious side effects include liver failure, metabolic acidosis, glaucoma, depression, diplopia, and speech and language disorders [26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






