6 The donor procedure
Introduction
The majority of organs transplanted over the last 40 years have come from donation after brain death (DBD) donors, but over the last decade the number of suitable donors has declined. As a result the number of living donors, particularly kidney, has significantly increased and now accounts for 35% in the UK and over 50% in the USA. In addition there are now an increasing number of expanded criteria donors (ECDs) and donation after cardiac death (DCD) donors. The terminology used for the different types of deceased donors is summarised in Table 6.1.
Table 6.1 Terminology used in deceased donation
| Preferred term | Other terms |
|---|---|
| Deceased donor | Cadaveric donor |
| Donation after brain death (DBD) | Heart-beating donor |
| Brainstem-dead donor | |
| Donation after cardiac death (DCD) | Non-heart-beating donor |
| Asystolic donor | |
| Extended criteria donor (ECD) | Marginal donor |
General issues
Legislation
The Human Tissue Act 2004 (HT Act) gives a framework for the removal, storage and use of tissues and organs from the deceased, and the storage and use of tissues and organs from the living, for specified health-related purposes, which includes donation for transplantation. The Human Tissue Act 20041 covers England, Wales and Northern Ireland, whilst the Human Tissue (Scotland) Act 20062 covers Scotland. Consent (or authorisation in Scotland) is the fundamental principle that underpins the Act. In deceased donation the wishes of the deceased in life should take precedence after death. Their wishes may have been expressed in life by being registered on the Organ Donor Register or by letting a family member know. If the wishes of the deceased are not known then consent should be obtained from a nominated person or a person in a qualifying relationship. It is important that trained individuals, be they intensive care staff or transplant coordinators, make the approach to the family. Further details can be found in the Codes of Practice on Consent3 and Donation of organs, tissues and cells for transplantation4 produced by the Human Tissue Authority (HTA). Internationally there are different legal approaches. In North America, as in the UK, it is the consent of the individual that is key, although there have been a number of modifications to the legal framework to allow an increase in organ donors. In Europe many countries have a legal framework based around presumed consent or opting out, and this approach is currently being debated in the UK.5
Consent for the removal of organs from living donors in the UK is covered by the common consent law, although consent for the storage and use is covered by the HT Act. Under the HT Act, the HTA regulates all living donor transplantation. Independent Assessors (IAs) are senior professionals trained and accredited by the HTA who act as representatives of both the donor and the HTA. They assess donor–recipient pairs to ensure the requirements of the HT Act are met and then recommend whether or not HTA approval should be given. Based on the online report submitted by the IA, the HTA executive is able to give approval for all straightforward directed genetically or emotionally related living-donor transplants, whilst a panel of HTA members need to approve all complex cases.
Organ retrieval
Organ retrieval is a stressful procedure for teams involved and specific stressors identified include emotional, technical, environmental/organisational, communication and long hours (Lorna Marson, Edinburgh, personal communication). In general, organ retrieval is not popular amongst consultant surgeons and consequently training and supervision is usually delegated to other trainees. This trend needs to be reversed in order to improve the quality of organs retrieved and training initiatives include an annual UK Transplant Organ Procurement Workshop and a separate module in the new surgical curriculum.5
Working groups from UK Transplant and the British Transplantation Society have produced recommendations for a national organ retrieval service and these are included within the report from the Organ Donation Taskforce.6 The chief principles of such a service are summarised in Box 6.1. These changes will come at a price, but if implemented will lead to a properly resourced and funded service that will benefit all involved (see Chapter 2). A pilot scheme has already been run in Scotland with good effect.7
Box 6.1 Principles of a national organ retrieval service
Organ preservation
Preservation of organs is an important factor in ensuring viability of the organ and in optimising outcomes. Once an organ no longer has a supply of oxygenated blood, cell damage will occur due to a depletion of adenosine triphosphate (ATP) and a failure of the sodium–potassium pump. This leads to swelling of the cell and anaerobic metabolism with the accumulation of lactic acid. This cellular acidosis leads to lysosomal damage and subsequent cell death. More detailed descriptions of this process of cell damage can be found in previous editions of this volume8,9 and elsewhere.10 Historically static cold storage has been the method of choice, but this may be suboptimal for the increasing number of ECDs and DCD donors. Alternative techniques such as hypothermic machine perfusion and normothermic perfusion have been investigated as means of improving preservation and consequently outcomes.10
Static cold storage
Cold storage is the preservation technique of choice in the majority of abdominal organ transplant units. This requires rapid intravascular flushing with cold preservation fluid to promote cooling of the organ, washout of blood components and rapid equilibration of the fluid with the tissues. Cooling results in a 50% reduction of the metabolic rate for every 10 °C fall in temperature, so that at a storage temperature of between 0 and 4 °C the metabolic rate is less than 10%. However, hypothermia alone will not completely prevent cell swelling and damage and the subsequent reperfusion injury, hence the use of a preservation fluid. There are a wide range of preservation fluids whose key components are an osmotic agent or impermeant to prevent cell swelling, a buffer to counteract the effect of intracellular acidosis, and an antioxidant to prevent the formation of reactive oxygen species. Preservation fluids currently in clinical use include Marshall’s or Hyperosmolar Citrate (Soltran®, Baxter Healthcare), University of Wisconsin (UW) solution (ViaSpan™, Bristol Myers Squibb), Histidine Tryptophan Ketoglutarate (HTK) solution (Custodial®, Kohler Medical Limited), EuroCollins (Fresenius) and Celsior® (SangStat Medical). A summary of the properties of each is detailed elsewhere.10 Further solutions are in development, which may help to reduce the ischaemia–reperfusion injury, but also benefit the marginal or extended criteria donor. These include Polysol, IGL-1 and AQIX® RS-1.10–12
 A large international multicentre analysis of kidney preservation in over 90 000 deceased donors showed that when the cold ischaemic time was less than 18 hours, there was no significant difference in risk of failure for the type of preservation fluid (UW, Marshall’s, HTK or EuroCollins) used. However, UW solution showed superior results when the cold ischaemic time was greater than 24 hours.13
A large international multicentre analysis of kidney preservation in over 90 000 deceased donors showed that when the cold ischaemic time was less than 18 hours, there was no significant difference in risk of failure for the type of preservation fluid (UW, Marshall’s, HTK or EuroCollins) used. However, UW solution showed superior results when the cold ischaemic time was greater than 24 hours.13
UW solution is the preferred choice of preservation solution in liver, pancreas and intestinal donors throughout the world. Preservation solutions used in heart and lung donation are discussed in more detail in Chapter 11.
Hypothermic machine perfusion
 A systematic and meta-analysis of the effectiveness of machine perfusion to cold storage demonstrated a 20% reduction in risk for DGF, but with no difference in 1-year graft survival. The need for studies of high methodological quality and sufficient size were recommended.14
A systematic and meta-analysis of the effectiveness of machine perfusion to cold storage demonstrated a 20% reduction in risk for DGF, but with no difference in 1-year graft survival. The need for studies of high methodological quality and sufficient size were recommended.14
In the multicentre Collaborative Transplant Study (CTS) analysis of kidney preservation, pulsatile machine perfusion was not superior to cold storage, although the numbers in the former group were small (n = 2200, or 2.6%).13 There are currently two multicentre randomised controlled studies taking place in Europe comparing cold storage with the LifePort machine. One study based in Belgium, The Netherlands and Germany is looking at DBD donor kidneys, and the other based in the UK (the PPART study) is looking at DCD donor kidneys. Recruitment is complete and the findings are due to be reported soon.
Normothermic perfusion
The normothermic perfusion of abdominal organs using a cardiopulmonary bypass system should allow the successful preservation of ECDs as well as permitting viability testing prior to transplantation. This has already been well proven in animal models, although there are logistical issues of using this in humans. A number of centres in the USA and Spain have used veno-arterial extracorporeal membrane oxygenation (ECMO) after cardiac arrest in the DCD group of patients to increase the numbers of kidneys and livers for transplantation.15,16 This technique restores the flow of warm oxygenated blood during the interval between death and organ procurement. This allows viability assessment and improves early function.
Preconditioning
 A randomised controlled trial of ischaemic preconditioning in liver transplantation improved post-transplant liver function, demonstrating that the technique protects against ischaemia–reperfusion injury.17
A randomised controlled trial of ischaemic preconditioning in liver transplantation improved post-transplant liver function, demonstrating that the technique protects against ischaemia–reperfusion injury.17
However, the use of pharmacological techniques raises ethical considerations as treatments are required in the donor that are not of direct benefit to the donor, but rather to the recipient.18
Organ damage
Donor organ disease increases with age and in the kidney includes changes due to hypertension and diabetes, whilst in the liver changes due to fatty infiltration are common. With a shortage of deceased-donor organs marginal organs are increasingly being considered, whereas not so long ago they would not have been. In addition infection and malignancy can be transmitted in the donor organ, making quality and safety one of the three key challenges facing transplantation as described in a recent European Union Policy document.19
Surgical damage is a potentially avoidable event, which can often be salvaged, but adds to the complexity of the recipient operation and can lead to complications. The largest published study looked at over 9000 kidneys retrieved in the UK.20 Repairable damage was recorded in 19% of cases and unsalvageable damage in 1%. One- and 3-year graft survival rates were not significantly different between repaired and undamaged organs. The lowest rates of damage were found in those retrieved by multiorgan retrieval teams performing more than 50 retrievals per year. This suggests that surgical training and experience are important and the implementation of a properly resourced national retrieval service should result in fewer surgically damaged organs. Further damage can also occur during benchwork of the organ and this has not been quantified.
The third cause of organ damage is ischaemia, which is classified as warm or cold ischaemia. There are different nomenclatures in use, but the following is suggested for universal use:21
 A large international multinational analysis of kidney preservation showed that cold ischaemia times up to 18 hours were not detrimental to graft outcome, but that the risk of graft failure increased beyond that with increasing ischaemic times. Within the first 18-hour period the time of ischaemia had no significant effect upon graft survival.13
A large international multinational analysis of kidney preservation showed that cold ischaemia times up to 18 hours were not detrimental to graft outcome, but that the risk of graft failure increased beyond that with increasing ischaemic times. Within the first 18-hour period the time of ischaemia had no significant effect upon graft survival.13
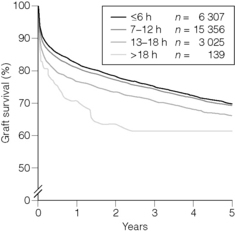
Similar data from the UK show that the cut-off time is 20 hours, after which there is an increased risk of kidney transplant failure (UK Transplant, unpublished data). In liver transplantation longer cold ischaemic times lead to poorer graft outcomes, although an analysis of the cut-off time has not yet been determined (Fig. 6.1). In DCD donors cold ischaemia times should be minimised and implantation performed as soon as possible.
Deceased donation
 The conclusions of the review were that multiorgan donation need not compromise the outcome of individual organ transplants, dissection of abdominal organs is better performed following cold perfusion, abdominal organs should be rapidly removed en bloc and separation performed on the back table in the cold; single aortic cannulation is superior to combined aortic and portal cannulation and increased aortic perfusion improves outcome.22
The conclusions of the review were that multiorgan donation need not compromise the outcome of individual organ transplants, dissection of abdominal organs is better performed following cold perfusion, abdominal organs should be rapidly removed en bloc and separation performed on the back table in the cold; single aortic cannulation is superior to combined aortic and portal cannulation and increased aortic perfusion improves outcome.22
Donor identification and assessment
The donor transplant coordinators fulfil a vital role in the early assessment of the donor (Table 6.2) and in liaison with the individual specialists assess the donor with regards to organ suitability for transplantation.
Table 6.2 Role of the donor transplant coordinator
| Standard | Aim | |
|---|---|---|
| 1 | Education | Develop and deliver educational strategies and clinical initiatives in order to raise and maintain the profile and awareness of organ/tissue donation to relevant healthcare professionals |
| 2 | Donor identification and referral | To ensure identification and referral of all potential organ/tissue donors to the DTC at the earliest opportunity in order to maximise donor management and outcome |
| 3 | Donor management/assessment process | To maintain respect and care of the donor ensuring that optimal donor management is implemented To minimise the risk of transmission of infections/diseases from the donor ensure that all relevant donor information is obtained to enable recipient centres to make an informed decision regarding organ suitability |
| 4 | Care of the donor family | To ensure the donor family is given clear and accurate information about organ/tissue donation enabling them to make a fully informed decision |
| 5 | The organ/tissue referral process | To ensure that organs/tissues are allocated in line with national policies |
| 6 | Care of the donor during the theatre process | To maintain the dignity and respect of the donor whilst continuing the coordination process including donor management to optimise the suitability of the donor organs and tissues being removed |
| 7 | Healthcare professional follow-up | To ensure that all healthcare professionals are given adequate and appropriate support |
All potential donors should be considered and discussed with the coordinator.6 There are general contraindications for donation as well as organ-specific criteria (Table 6.3). With the shortage of organs and successful use of organs from ECDs, some of these contraindications may be relative and have been challenged.
Table 6.3 Contraindications to organ donation
| General absolute contraindications to donation | |
| Infective | HIV/AIDS or HIV high-risk category (even if HIV antibody negative) |
| Severe sepsis | |
| Viral encephalitis | |
| Recipients of human growth hormone or risk of nvCreutzfeldt–Jacob disease | |
| Malignant | Concurrent or recent malignancy (excluding CNS malignancy except glioblastoma multiforme) |
| (Recent generally refers to solid-organ malignancy within the last 5 years) | |
| Organ-specific contraindications | |
| Heart | Established cardiac disease |
| Previous cardiac surgery | |
| Prolonged cardiac arrest | |
| Myocardial infarction | |
| Irreversible poor cardiac output | |
| Age >60 | |
| Abnormal chest X-ray (minor changes acceptable and occur in 27% of donors) | |
| Abnormal 12-lead ECG | |
| High inotrope requirement (above <10 μg/kg/min dopamine to maintain systolic blood pressure >90 mmHg if euvolaemic) | |
| Lungs | Age >60 |
| Established lung disease | |
| Bronchopneumonia | |
| Heavy smoker | |
| Pulmonary aspiration and tracheal colonisation with bacteria and fungi | |
| Parenchymal trauma | |
| Previous lung surgery | |
| Irreversible poor gas exchange | |
| Abnormal chest X-ray | |
| Inadequate gas exchange (PaO2 <50 kPa on 100% O2 with 5 mmHg peak end-expiratory pressure) | |
| Liver | Established liver disease |
| Inborn error of metabolism (liver based) | |
| History of alcohol abuse (note: liver function tests can be deranged secondary to hypotension/asystole) | |
| Kidneys | Age not strictly limited (although <75 years recommended) |
| Established chronic kidney disease | |
| Pancreas | Age <50 years |
| No history significant cardiovascular disease | |
| No history alcohol abuse | |
| No major obesity | |
| No type II diabetes |
Diagnosis of death
There is no legal definition of death in the UK, although a generally accepted definition is the irreversible loss of the capacity for consciousness, combined with irreversible loss of the capacity to breathe.23 Details on the diagnosis of brainstem death and cardiac death can be found in the Code of Practice for the Diagnosis and confirmation of Death.23
Donor management
Physiology of brain death
The events preceding brain death, for example trauma or intracranial surgery, may impact on the quality of the retrieved organs. There are also significant physiological changes associated with brain death that affect each of the organs. This has been investigated both in controlled animal models and in human donors. Initially at the point of brain death the brainstem compression associated with coning results in hypertension and bradycardia, the Cushing reflex, which lasts about 15 minutes. This is followed by the autonomic storm in which massive catecholamine drive occurs leading to a transient hyperdynamic state,24 although end-organ hypoperfusion can arise due to blood shunting. The pituitary hormones vasopressin and adrenocorticotropic hormone (ACTH) decline from 15 to 45 minutes, as do tri-iodothyronine, thyroxine and glucagon. This results in transient hypertension and also in hypoperfusion of organs. Insulin and lactate dehydrogenase show a moderate increase and diabetes insipidus is common.25
The effect on the cardiovascular physiology is to increase left and right ventricular end-diastolic pressure, increase cardiac output, and decrease systemic and pulmonary vascular resistance. This is transient and after 2–4 hours right and left ventricular systolic function deteriorates significantly, with the right ventricle being affected more than the left.26
The liver is also affected by hypotension and hypoperfusion; and human studies support the principle that brainstem death causes activation of inflammatory mediators within the liver and subsequently results in increased injury upon reperfusion.27
In the kidney cardiovascular events may result in tubular injury alone, although studies in human donor organs have implied that brain death produces both non-specific endothelial damage and also increases organ immunogenicity.28 These effects may be reduced with proper donor management.29
Clinical management
The principles of donor management are to provide cardiovascular stability and maintain organ function. The first principle is adequate fluid management and although inotropes are often required their use during donor maintenance should be minimised.30 The goal of management is to maintain blood pressure at a mean arterial pressure of >60–70 mmHg, with the inotrope of choice being vasopressin.31 It acts as a vasopressor with no β-adrenergic response and also has an antidiuretic effect. This is also of benefit as diabetes insipidus (DI) occurs in two-thirds of brainstem-dead donors and further contributes to fluid imbalances, although 1-D-amino-8-D-arginine vasopressin (DDAVP) may be required if profound DI occurs. Other inotropes are limited in their use. High-dose adrenaline can be detrimental to donor organs as local vasoconstriction occurs, and the vasodilator effects of dobutamine may lead to undesirable hypotension and tachycardia.32 The use of thyroxine replacement is controversial, although most guidelines support its use as it increases myocardial contractility due to β-adrenergic receptor- independent processes.33 Glycaemic control is often deranged in the brain-dead donor and insulin infusion may be required to maintain the blood glucose within a normal range.
 A summary of the medical management of the organ donor based on the Intensive Care Society guidelines for Adult Organ and Tissue Donation34 is shown in Table 6.4.
A summary of the medical management of the organ donor based on the Intensive Care Society guidelines for Adult Organ and Tissue Donation34 is shown in Table 6.4.
Table 6.4 Summary of donor management (based on Intensive Care Society guidelines34)
| Clinical problem | Management |
|---|---|
| Endocrine | |
| Diabetes insipidus | Maintain sodium at 155 mmol/L with 5% dextrose |
| Maintain urine output at 1–2 mL/kg/h with vasopressin | |
| If vasopressin fails to control diuresis, intermittent desmopressin (DDAVP) may occasionally be required | |
| Hyperglycaemia | Insulin infusion to maintain plasma glucose 4–9 mmol/L |
| Hypothyroidism | Tri-iodothyronine (T3) |
| General stability and reduction of inflammatory response | Methylprednisolone |
| Cardiovascular support | |
| Decreased mean arterial pressure with increased cardiac output | Preload optimisation and vasopressor to increase afterload |
| Decreased mean arterial pressure with decreased cardiac output | Preload optimisation, vasopressor to increase afterload and inotrope to increase contractility |
| Increased mean arterial pressure with decreased cardiac output | Preload optimisation and vasodilator to increase afterload ± inotrope to increase contractility |
| Respiratory | |
| Maintenance of normocapnia (PaCO2 ≈︀ 5.0–5.5 kPa) | Consider pressure control ventilation |
| Modes that allow patient triggered ventilation are not appropriate | |
| Ventilation with the lowest FiO2 to maintain PaO2 >10.0 kPa | Very sensitive ventilatory triggers may allow cardiac cycle-induced pressure changes to trigger the ventilator. This may cause diagnostic confusion by giving the appearance of a spontaneous breath |
| Peak end-expiratory pressure >5 cmH2O may reduce cardiac output and is rarely required | |
| High inspiratory pressures should be avoided | |
| Haematological support | |
| Haemoglobin concentration should be maintained over 9 g/dL | Blood transfusion |
| Deranged coagulation | Fresh frozen plasma and platelets |
Surgical management and operative procedure
The procedure must be exercised in a professional manner. Currently the donor procedure is often performed out of hours with teams that may not work regularly together, in unfamiliar hospital environments. Separate teams for the thoracic and abdominal organ retrieval are normal and multiple teams including pancreas and renal teams may also be involved.
The operating surgeons should be experienced in retrieval surgery and in dealing with the challenges of difficult retrievals. Damage to retrieved organs is not uncommon, with studies estimating injuries to be up to 19%.20 Donors who are obese, paediatric and with intra-abdominal trauma or previous surgery provide significant surgical challenges, calling on a high level of surgical expertise if injury is to be avoided.
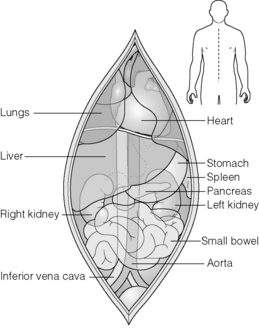
Techniques vary between surgeons and regions but often the procedure will begin with a full midline laparotomy with a thorough inspection of the intra-abdominal viscera to exclude pathology, particularly occult neoplasms (Fig. 6.2). Following this the intra-abdominal team proceed to mobilise the right colon and small bowel to expose the retroperitoneum. The dissection extends to the level of the superior mesenteric artery. This exposes the vena cava and renal veins and allows careful assessment of accessory renal vessels. The iliac arteries or lower aorta are then carefully encircled and controlled with heavy ties. These are used to secure the cannulas prior to cold perfusion. The ureters may be identified at this stage but care must be taken to ensure they are not devascularised if dissected. The portal vein may also be cannulated indirectly via the superior or inferior mesenteric veins, although this is not practised universally and may be unnecessary or detrimental if the pancreas is also retrieved.35,36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree