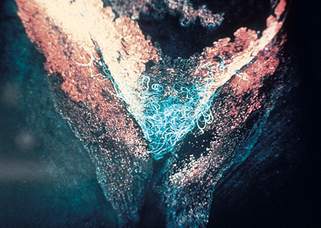Chapter 3 Diagnostic techniques
1. What is the most sensitive office laboratory test for diagnosing dermatophyte infections of the skin?
Microscopic examination of a potassium hydroxide (KOH) preparation of scrapings taken from the affected area is the most sensitive office laboratory test. A study of 220 specimens examined by both KOH and culture demonstrated positive KOH preparations and positive cultures in 45% of samples, a positive KOH preparation and negative culture in 52% of samples, and a negative KOH preparation and a positive culture in only 3% of samples. However, cultures can be useful because other studies have shown a 5% to 15% increase in positive specimens by culturing KOH-negative materials. Moreover, it is important to realize that the diagnostic accuracy of the KOH preparation depends on the skill of the individual performing the test.
2. How is a KOH examination performed?
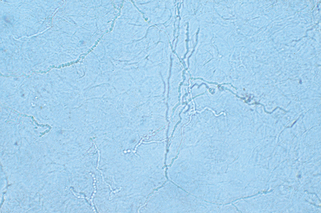
The highest rate of recovery of organisms occurs in specimens taken from the tops of vesicles, the leading edges of annular lesions, or deep scrapings from the nails suspected to be infected with fungi. The site should be swabbed with an alcohol pad or water and scraped with a no. 15 blade. In some instances, such as the scalp or nail, a curette may be more effective. The moist corneocytes are then easily transferred from the blade to a glass slide. One or two drops of KOH (10% to 20%) are added, and a coverslip is applied to the specimen. The KOH preparation is gently warmed, but not boiled, and then examined under the microscope. It is important to focus back and forth through the material so that the refractile hyphae can be visualized. Fungal hyphae can be recognized by their regular cylindrical shapes with branching and the presence of septae that may demonstrate a subtle greenish hue (Fig. 3-1). A pencil eraser or pen cap gently applied to the surface of the coverslip may enhance keratinocyte breakdown, especially for clumped specimens. If no organisms are observed initially, waiting 10 to 15 minutes may aid visualization.
3. What laboratory tests are useful for diagnosing tinea capitis?
Testing for fluorescence in the affected area using a Wood’s light is the quickest technique. If the hair fluoresces yellow-green, then a fungal infection is likely. However, lack of fluorescence does not exclude tinea capitis, because Trichophyton tonsurans accounts for 80% to 95% of scalp ringworm infections in the United States, and it does not fluoresce. Therefore, examination of KOH-treated infected hair is more sensitive and can also be rapidly performed. The best results are obtained when broken-off hairs are examined, because these are the ones infected by hyphae and arthrospores. Most dermatophytes, such as T. tonsurans, grow within the hair shaft (endothrix), and a few minutes are required to let KOH break down the hair shaft and visualize the infection. Finally, the diagnosis can also be proved by fungal cultures. The easily broken, infected hairs are embedded in the media. The specimen can be obtained using a no. 15 blade, curette, or hemostat.
4. What is a Wood’s light or lamp? How is it useful in skin diseases?
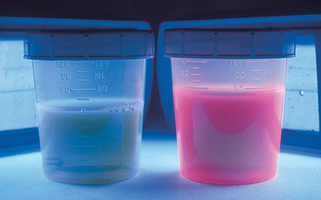
A Wood’s light produces invisible long-wave ultraviolet radiation, or “black light,” at a wavelength of 360 nm. When this light strikes the surface of the skin or urine, fluorescence is produced in some disorders. This fluorescence is best observed in a completely dark room. The Wood’s lamp is useful in diagnosing cases of several skin conditions: tinea capitis (see preceding text), tinea versicolor (dull yellow fluorescence), erythrasma (coral red fluorescence; Fig. 3-2), and Pseudomonas infections of the skin (green fluorescence). It is also useful as a screening test in porphyria cutanea tarda as the urine fluoresces a coral red color (Fig. 3-3). The Wood’s light may also be used in certain disorders of pigmentation. In patients with hyperpigmentation, it is used to localize the site of the pigment because it accentuates superficial epidermal pigment, whereas deeper dermal pigment is unchanged. It is also used in patients with vitiligo because it demonstrates complete depigmentation. Finally, it can be used to delineate the borders of melanocytic lesions, such as lentigo maligna, prior to surgery.
5. Name common culture media used for isolating dermatophytes.
Dermatophyte test media (DTM) and Sabouraud’s dextrose agar, with or without antibiotics (e.g., Mycosel agar, Mycobiotic agar), are the two most common types of culture media used. Many dermatologists prefer DTM because it has the advantage of a color indicator that changes the media from yellow to red when a dermatophyte is present. DTM is 95% to 97% accurate in differentiating dermatophytes from nondermatophytes. Sabouraud’s dextrose agar is a standard in mycology laboratories and also in many dermatologists’ offices. It consists of dextrose (energy source), peptone (protein source), and agar (for a firm surface). Antibiotics can be added to suppress bacterial contaminants, and cycloheximide is added to suppress yeasts and nondermatophytes. Plain Sabouraud’s agar is an especially good culture medium for Candida albicans.
Head E: Laboratory diagnosis of the superficial fungal infections, Dermatol Clin 2:93–108, 1984.
6. Describe a simple test for tinea versicolor other than a KOH preparation.
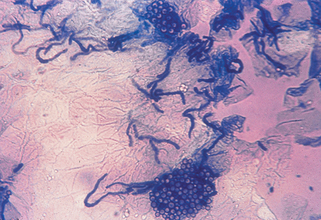
Clear cellophane tape preparations are an excellent diagnostic testing material because the organism is found in the upper stratum corneum. First, the skin is scraped to ensure there is adequate scale. The tape is applied over the scale and then mounted on a glass slide and examined under the microscope. Clusters of short hyphae and yeasts are seen producing a “spaghetti and meatballs” pattern. Methylene blue may also be added to the slide, selectively staining the organism and thus enhancing visualization (Fig. 3-4). It is important to note that Malassezia globosa and M. furfur, the most common etiologic agents of tinea versicolor, cannot be cultured on any of the routine fungal media kept in most laboratories.
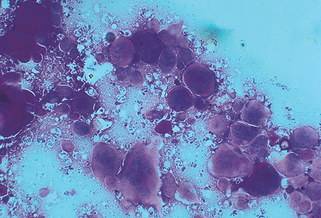
7. What is a Tzanck preparation or smear?
A Tzanck smear is a standard technique for the rapid diagnosis of herpes simplex virus (HSV) or varicella-zoster virus (VZV) infections. It cannot distinguish between these two agents, nor can it distinguish between HSV subtypes (HSV type 1 or 2). It is performed by scraping the base of a fresh blister with a scalpel blade and then spreading the adhering cells and material onto a glass slide. The slide is then stained with a Giemsa, Wright, or Sedi stain. The typical multinucleated giant cells or atypical keratinocytes with large nuclei are then easily visualized (Fig. 3-5).




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree