(1)
Obesity Institute, Geisinger Medical Center, Southold, NY, USA
Abstract
The evolution of bariatric surgery has been a major factor in the return of endoscopy to the scope of practice and training in general surgery. Endoscopy is an essential specialty component of bariatric surgery programs. Interventional endoscopy is now emerging as a treatment option in the nonoperative management of complications of bariatric surgery. This chapter reviews current indications, controversies, and best practice recommendations regarding endoscopy in the perioperative period.
Gastrointestinal surgeons have embraced upper gastrointestinal endoscopy as a diagnostic and therapeutic tool, and its use by surgeons in the evolution of bariatric surgery has been a major factor in promoting the establishment of these procedures in the scope of general surgery clinical practice and training. Since the development of bariatric surgery, flexible upper endoscopy has been an important tool for patient management, both before and after bariatric surgery. During recent years, endoscopic interventions for both weight loss and treatment of metabolic disease have been introduced and are being incorporated into treatment protocols by bariatric surgery centers. The purpose of this chapter is to review the current practice guidelines and evidence concerning the use of flexible endoscopy in bariatric surgery.
Preoperative Endoscopy
Endoscopy is commonly utilized in the preoperative evaluation of candidates for bariatric surgery. Many patients who suffer from extreme obesity have symptomatic disease of the foregut as a comorbid condition, and in the presence of foregut symptoms such as dyspepsia and gastroesophageal reflux, the indications are established. If the contemplated bariatric surgical procedure is a gastric bypass or biliopancreatic diversion with duodenal switch, the distal stomach and duodenum will be inaccessible by conventional endoscopy, thereby raising concern about missing important pathology. The purpose of preoperative endoscopy is to detect and treat lesions that potentially can affect the type of surgery performed, cause postoperative complications, or contribute to symptoms later after surgery [1]. There are a number of published series of upper endoscopy in candidates for bariatric surgery which demonstrate a variety of foregut conditions like large hiatal hernias, acute ulcers of the stomach or duodenum, esophagitis, and Barrett’s Esophagus often in patients without symptoms of foregut disease [2–6]. Not infrequently, lesions discovered at endoscopy result in a change of surgical approach or a delay in surgery for appropriate treatment or additional studies (Table 10.1) [2–7].
Table 10.1
Lesions discovered at endoscopy prior to bariatric surgery and their potential impact on surgical decision-making
Lesions | Frequency (%) | Impact |
|---|---|---|
0–23 | Delay surgery for healing; repeat endoscopy | |
<10 | Avoid band placement; consider crural repair | |
3 | Delay surgery for medical treatment | |
0.2–3.1 | Postoperative surveillance | |
Gastric cancer [7] | 1 case report | Gastric resection |
<1 | Gastric resection or survey remnant stomach | |
<1 | Surveillance of gastric remnant or resection | |
Gastric polyps [5] | <1 | Surveillance of gastric remnant or resection |
Gastric MALT lymphoma [24] | 1 case report | Medical treatment and/or resection |
Helicobacter pylori | 30–40 | Medical irradiation |
European guidelines for bariatric surgery recommend preoperative endoscopy for all patients prior to surgery, even those without symptoms [8].
The important benign, premalignant, and malignant conditions encountered in bariatric surgery have been recently reviewed [2]. Barrett’s Esophagus is more common in obesity [9, 10], and has been reported as an endoscopic finding with a frequency approximating 1–3.7 % in bariatric surgery candidates [3, 11, 12]. Preliminary results suggest that this lesion will regress or disappear after gastric bypass [13]. The implications of knowledge of this condition before bariatric surgery on the informed consent process, the choice of surgical procedure, and on post-op surveillance are obvious.
There is also an association of adenocarcinoma (as opposed to squamous carcinoma) of the esophagus with obesity [14]. Although this lesion has not as yet been reported in candidates for bariatric surgery, there are a number of case reports of this lesion developing as early as several months after bariatric surgery raising the question of whether or not the lesion was present before the surgery [15, 16]. There is no known association between gastric cancer and obesity, but gastric cancer has been identified in at least one candidate for bariatric surgery [7].
Gastrointestinal Stromal Tumors (GIST) can be incidentally encountered during bariatric procedures [17]. These lesions are nearly always asymptomatic and are unlikely to be discovered at preoperative endoscopy. They are usually found in the gastric fundus and cardia and may be missed unless the surgeon inspects the stomach during laparoscopy. When encountered, a frozen section should confirm the diagnosis and the lesion should be resected with clear margins (R0 resection).
Another lesion of concern to the bariatric surgeon is intestinal metaplasia, which occurs in evolution after atrophic gastritis and can slowly progress to gastric adenocarcinoma. This lesion has been found on preoperative endoscopy in several series of bariatric surgery candidates [18, 19], many of whom were asymptomatic [19], and has been found on histological examination of 427 consecutive resected gastric remnants after gastric bypass in 0.7 % of patients [20]. In the presence of extensive intestinal metaplasia of the incomplete type in the distal stomach, resection at the time of gastric bypass may be indicated [21].
Additional lesions that are important to bariatric surgeons include carcinoid tumors, gastric polyps, and gastric lymphoma. Carcinoid tumors are neuroendocrine tumors that occur rarely (4 %) in the stomach [2]. These lesions have been found occasionally in the stomach [22] and duodenum [3] on endoscopic evaluation of bariatric surgery candidates. When multiple gastric carcinoids are encountered, gastric resection should be a consideration in conjunction with the bariatric procedure [22]. Gastric polyps are also occasionally found on preoperative endoscopy [5]. Of these, adenomatous polyps are considered premalignant, although hyperplastic and hamartomatous polyps are rarely associated with gastric cancer. When these are encountered, consideration regarding gastric resection at the time of bariatric surgery or surveillance of the gastric remnant is necessary [23]. There is one case report of a gastric lymphoma identified on preoperative endoscopy in a bariatric surgery candidate [24].
Helicobacter pylori (H. pylori) infection is present in 30–40 % of candidates for bariatric surgery, and studies suggest that preoperative testing may be advantageous. Treatment to eradicate H. Pylori prior to bariatric surgery was found in one study to reduce the incidence of postoperative marginal ulcers when compared with historical controls [4]. In another study, a positive test for H. Pylori was associated with a higher likelihood of positive endoscopic findings [19]. Preoperative testing for H. Pylori, and eradication if found positive, is recommended for bariatric surgery candidates [1].
Available information regarding risk and outcomes of endoscopy in extreme obesity is limited. Emerging evidence suggests that increasing BMI is a risk factor for hypoxemia during endoscopy [25]. In one small series of preoperative endoscopies in candidates for bariatric surgery, critical events occurred as severe hypoxemia (SaO2 < 60 %) occurred in 2.9 % and were associated with sleep apnea syndrome. Emergency bronchoscopic intratracheal insufflation with oxygen was required in each case [6]. Endoscopy in the severely obese carries significant risk, and better studies are needed to define risk factors. Clearly sleep apnea and super obesity may be indications for anesthesia support for endoscopy [26].
Despite the fact that foregut lesions may be found on surveillance endoscopy in asymptomatic bariatric surgery candidates, routine preoperative use of endoscopy remains somewhat controversial. In asymptomatic patients, the likelihood of finding a lesion which will change the surgical approach is low, and, similar to screening mammography, may result in additional negative testing which adds to the cost and does not alter the management. Additional prospective studies are needed to assess the outcome benefits of routine preoperative endoscopy in bariatric surgery.
Postoperative Endoscopy
Endoscopy has a major role in the postoperative managements of patients undergoing bariatric surgical procedures. As more bariatric surgeons are trained in the era of minimally invasive surgery, they are performing endoscopy on their own patients. The bariatric surgeon is in the best position to understand the postoperative foregut anatomy, and thus may have an advantage in the interpretation of the endoscopic findings. If non-surgeons perform postoperative endoscopy, they must be familiar with the anatomy of bariatric procedures, and should review operative notes and procedure diagrams (Figs. 10.1, 10.2, and 10.3). Foregut symptoms such as nausea, vomiting, and abdominal pain are symptoms that are common after bariatric surgery (Table 10.2).
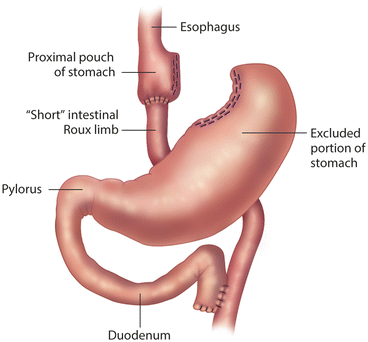
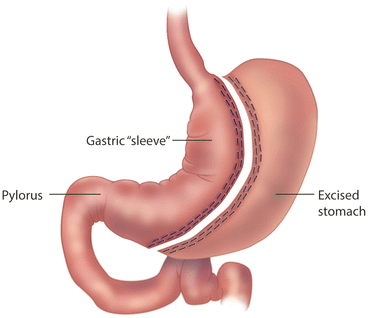
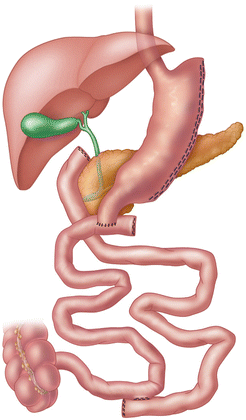

Fig. 10.1
Roux-en-Y Gastric Bypass

Fig. 10.2
Sleeve gastrectomy

Fig. 10.3
Biliopancreatic diversion with duodenal switch
Table 10.2
Clinical signs and symptoms that frequently lead to foregut endoscopy in postoperative bariatric surgery patients
• Nausea |
• Vomiting |
• Dysphagia |
• Pain |
• Reflux |
• Severe diarrhea |
• Anemia, bleeding |
• Weight loss failure |
Nausea, vomiting, and reflux symptoms commonly occur during the postoperative progression of diet, especially with the initiation of solid food. This often results in calls to the bariatric program from concerned patients. The most common cause of these symptoms in the early postoperative period is maladaptive eating behavior as patients struggle to adjust dietary habits to their new foregut anatomy by eating too much at one time, eating too fast, or eating with inadequate chewing. These complaints should mandate dietary counseling in conjunction with adjustment of diet to liquids followed by slow re-progression of diet. Symptoms that persist or progress despite dietary instruction and adjustment or symptoms occurring with foods that were previously well tolerated should be evaluated by endoscopy to look for organic causes [1, 27].
Several longitudinal cohort studies report that 7–11 % of bariatric surgery patients undergo postoperative endoscopy [28, 29]. A report of 1,079 gastric bypass patients from a major bariatric center over a 7-year period identified 76 patients (7 %) who were referred for endoscopy because of foregut symptoms. The endoscopic findings from this study are shown in Fig. 10.4 [29]. In this study, a normal endoscopy was found in 31.6 %, an observation confirmed in another study cohort revealing normal exams in 28 % of symptomatic postoperative patients [30]. A normal endoscopy in a symptomatic patient suggests that noncompliant eating behavior is the likely cause of symptoms, and that additional counseling and possible psychological consultation are needed. Often close questioning will identify sources of stress or tension in the home environment that cause the symptoms. Marginal ulcers are a common cause of foregut symptoms and are well-known surgical complications after bariatric surgery (Table 10.3).
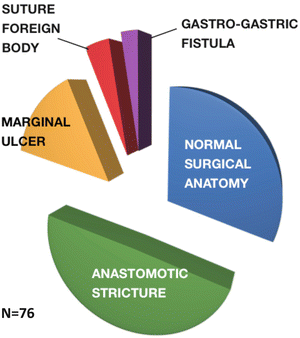

Fig. 10.4
The endoscopic findings in a series of 76 patients who underwent diagnostic endoscopy because of foregut symptoms after Roux-en-Y gastric bypass surgery. Modified from Lee J, Van Dam J, Morton J, Curet M, Banerjee S. Endoscopy is Accurate, Safe, and Effective in the Assessment and Management of Complications Following Gastric Bypass Surgery. Am J Gastroenterol. 2009; 104:575–582 [29]
Table 10.3
Foregut complications after bariatric surgery
• Marginal ulcer |
• Leak/fistula |
• Anastomotic stricture |
• Band erosion |
• Biliary tract disease |
The overall incidence of marginal ulcer is reported as 0.6–16 % in recent reviews [27, 31]. Several longitudinal studies have observed a decreasing incidence with time after bariatric surgery with the greatest incidence early in the postoperative period [30, 31]. The exact cause of marginal ulcer is not clear but proven risk factors include diabetes, tobacco use, nonsteroidal anti-inflammatory agent use, inhaled steroid use, and large gastric pouch size [31]. Additional contributing factors include mucosal ischemia, H. Pylori infection, foreign body, and gastrogastric fistula. The gastrojejunal anastomosis after roux-en-Y-gastric bypass is sensitive to gastric acid in the absence of duodenal bicarbonate buffering, and both increased pouch size and gastrogastric fistula will increase the potential for acid injury. If a marginal ulcer is found at endoscopy, the pouch must be carefully examined for gastrogastric fistula. If not found at endoscopy, an upper GI contrast study with dilute barium is indicated (Fig. 10.5a, b).
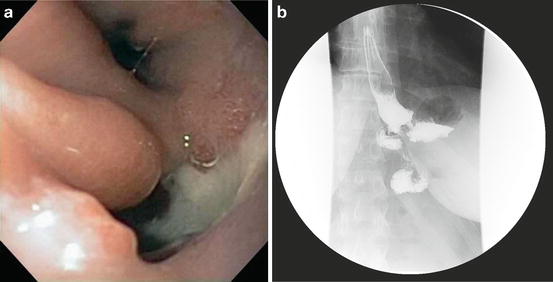

Fig. 10.5
(a) Endoscopic view of the gastric pouch and a gastrogastric fistula complicating a Roux-en Y-gastric bypass procedure (graciously provided by Jon Gabrielsen MD, Department of Surgery, Geisinger Medical Center, Danville Pa). (b) Upper GI view of a gastrogastric fistula after a Roux-en-Y gastric bypass procedure. Courtesy of Jon Gabrielsen MD, Department of Surgery, Geisinger Medical Center, Danville PA
Treatment of marginal ulcer includes proton pump inhibitors and sucralfate as well as smoking cessation. Ulcers that are refractory to medical treatment should be treated surgically. Factors that predispose to failure of medical treatment include gastrogastric fistula and tobacco exposure. A report of a refractory ulcer patient with a positive urinary nicotine level who denied tobacco exposure suggests that this scenario should be considered before surgical management takes place [30].
Stomal stenosis or anastomotic stricture is another fairly common complication of bariatric surgery, most often following the gastric bypass procedure. In the gastric bypass procedure, the surgeon must create a watertight and tension-free anastomosis with satisfactory blood supply. The anastomosis must be small enough to cause food restriction, but substantial enough to allow normal hydration and nutrition. The normal anastomosis diameter is usually about 12 mm. A stricture is defined as an anastomosis <10 mm in diameter [1]. This complication was reported with an incidence of 11–16 % [32–34] early in the evolution of laparoscopic gastric bypass, but more recent studies report an incidence of 4.4–8 % [35–38]. Factors contributing to stricture formation include mucosal ischemia, anastomotic tension, marginal ulcer with cicatrix formation, and factors related to anastomotic technique. Evidence suggests that the incidence is higher when the 21 mm circular stapler is used for the gastrojejunal anastomosis [38], but some have reported good results with this technique [36]. The lowest reported incidence (0.7 %) has been reported in a series with hand-sewn gastrojejunal anastomoses, using absorbable monofilament suture material [35].
This condition presents with vomiting and dysphagia initially with solids, but later involving liquids. Epigastric and retrosternal pain may also be present. Prompt diagnosis and treatment is essential in order to minimize the associated risk of problems with hydration or nutrition. The diagnosis can be made by contrast radiography, but endoscopy is preferred for more precise diagnosis, recognition of marginal ulcer, and treatment [1]. Endoscopic dilatation is the treatment of choice using balloon dilators or wire-guided boogie dilators. Gradual dilation over several endoscopy sessions is the most common approach in order to minimize the risk of perforation. Endoscopic dilatation in 1–4 sessions is successful in resolving the problem for most patients [1, 27, 36, 39, 40]. Patients who recur or fail to respond to multiple dilatations will require surgical revision[39]. Once the diagnosis of anastomotic stricture is made, concurrent nutritional care with alternate feeding strategies such as liquid nutrition or enteral nutrition via remnant gastrostomy may be needed.
In gastric bypass patients who have a retro colic Roux-en-Y limb, scar formation at the mesocolon opening may obstruct the Roux limb 10–15 cm from the gastrojejunal anastomosis [41]. The clinical presentation is similar to the presentation with gastrojejunal anastomotic stricture. Endoscopy will demonstrate a normal gastrojejunal anastomosis, a small segment of dilated jejunum, then the stricture. Treatment for this condition is surgical revision and not endoscopic dilatation [42]. The diagnosis of this complication is another example of the potential benefit of the bariatric surgeon performing his own postoperative endoscopy.
Gastrointestinal leaks and fistulas are life-threatening complications of bariatric surgery. The incidence from selected series from the last decade reported in a recent review ranges from 0.8 to 7 % [43]. More recent reports from large clinical registries from centers of excellence include leak rates of 0.42 [44] and 0.6 % [45]. The traditional management of leaks complicating bariatric surgery has been urgent surgery because of the common progression of systemic toxicity, multiorgan failure, hemodynamic instability, and death, which accompany leaks. As minimally invasive bariatric surgical procedures developed, the routine use of closed suction drains and early postoperative upper GI contrast studies has become common, and patients with postoperative leaks have been identified in a setting of stable vital signs and only mild foregut or respiratory symptoms [46]. Many of these patients have contained leaks defined by extra luminal contrast, which drains back into the GI tract, collects in small spaces, or immediately traverses the surgical drain [43]. Such patients may not need emergency surgery as a life-saving intervention, and may respond to nonoperative treatment, which includes nothing by mouth, intravenous antibiotics, drainage of all collections, and nutritional support.
Nonoperative management of leaks avoids the morbidity of emergency reoperation, but is a slow process necessitating a lengthy hospitalization. As nonoperative management of contained leaks became more widely practiced with success, endoscopic treatments involving the use of stent placement [47] and injection of fibrin sealants [48] have been introduced in an effort to accelerate healing with nonoperative treatment. Both endoscopic techniques can potentially seal the leak and allow for resumption of oral feedings and reduced peritoneal spillage. Although these techniques remain unproven as yet by randomized prospective trials, their efficacy in selected patients is established [49–52], and the use of nonoperative management of leaks, especially from the gastrojejunal anastomosis in gastric bypass and from the greater curve staple line in sleeve gastrectomy, is increasing [43, 51, 53]. Leaks from the gastric remnant and from more distal small intestinal anastomoses are more commonly associated with fulminant peritonitis, and emergency surgery remains the preferred treatment [42, 51, 53].
Additional endoscopic procedures recorded in case reports or small case series include endoscopic full-thickness gastric suturing to close small gastrogastric fistulae [54], as well as endoscopic vacuum-assisted sponge closure [55], and use of a fistula plug [56], or combinations of interventions in the treatment of leaks or fistulas. There is a need for prospective studies to establish efficacy and specific indications for these techniques, but it is apparent that new endoscopic treatment paradigms for management of leaks are emerging. As bariatric surgeons become more familiar and comfortable with nonoperative management of leaks and fistulas, they should be aware of the emerging body of knowledge involving the significance of the Gut Microbiota in the pathogenesis and management of sepsis complicating gastrointestinal surgery [57]. Since adult hypoalbuminemic malnutrition is common among bariatric patients treated for leaks with nonoperative therapy [50], and a risk factor for bad outcomes in gastrointestinal surgery [58], enteral nutritional support via gastrostomy, jejunostomy, or endoscopically placed nasojejunal feeding tube should be utilized as supportive treatment.
Gastric band erosion into the gastric lumen is another complication of bariatric surgery where endoscopy has a major role (Fig. 10.6a, b). Several recent studies report an incidence of <5 % with some variation in case series incidence up to 9 % from earlier reports [59–62]. Erosions can occur in the absence of symptoms, or cause loss of satiety, port site infection, and abdominal pain [60]. The diagnosis is made at endoscopy, and the treatment is removal with a subsequent revision bariatric surgery procedure. Removal is most commonly performed as a laparoscopic surgical procedure with band removal and gastric repair [59, 60, 63]. Techniques for endoscopic removal of eroded bands have been developed and carried out successfully in several small studies [61, 64]. Each approach to band removal has its own advantages and complications [65]. Both procedures require general anesthesia, and the endoscopic approach requires advanced endoscopic skills. The most cost-effective approach should be determined by prospective randomized trials.
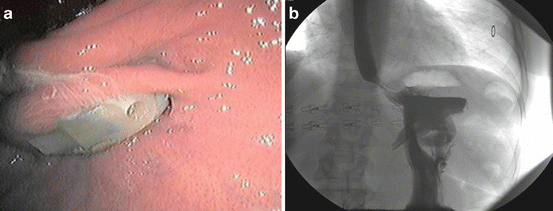

Fig. 10.6
(a) Endoscopic view of a gastric band eroding into the gastric lumen (Courtesy of Raul Rosenthal MD, Chairman, Department of General Surgery, Cleveland Clinic, Weston, Florida). (b) Upper GI series views of an eroded gastric band (Courtesy of Raul Rosenthal MD, Chairman, Department of General Surgery, Cleveland Clinic, Weston, Florida)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








