Protein
Synonyms
Location
Function
Factor XIIIa
Fibrin stabilizing factor
Platelets, chondrocytes, other cells
Blood Coagulation
TG1
Keratinocyte TG
Keratinocytes, brain
Keratinocyte differentiation
TG2
Tissue TG
Ubiquitous
Multiple functions
TG3
Epidermal TG
Squamous epithelium, brain
Hair follicle differentiation
TG4
Prostate TG
Prostate
Decreased immunogenicity of sperm
TG5
TGx
Ubiquitous
Cellular differentiation
TG6
TGy
Unknown
Unknown
TG7
TGz
Ubiquitous
Unknown
In the gut, tissue transglutaminase deamidates the insoluble wheat protein, gliadin, altering its solubility as well as its immunogenicity. In patients with celiac disease, antibodies are generated against both gliadin as well as the tissue transglutaminase protein [22]. These IgA antibodies can be detected in the serum in a high percentage of celiac disease patients [95–98 %] and a slightly lower percentage of patients with DH [75 %] [23]. In patients with DH, perhaps through a process of epitope spreading, antibodies against epidermal transglutaminase are also present and can be detected in the granular deposits of IgA in the dermal papillae [24]. These anti-epidermal antibodies can be measured in the serum of 95 % of DH patients not on a gluten free diet [25]. Antigen-antibody complexes deposit in the dermal papillae of the skin, which leads to neutrophil recruitment, activation, and subsequent destruction of structural proteins present at the dermoepidermal junction which leads to sub-epidermal cleft formation [26].
Clinical Presentation
The classic clinical presentation of patients with DH is that of a papulovesicular eruption that is present on the extensor surfaces of the extremities, sacrum, and scalp (Fig. 10.1). Pruritus is significant, often dramatically affecting quality of life, and can be confined to the specific skin lesions or generalized. Due to the degree of pruritus that is present, the classic skin lesions of papulovesicles are often difficult to find as secondary changes of scratching such as excoriations or dermatographism may be the predominant clinic findings (Fig. 10.2). With more advanced cutaneous disease, the papulovesicles can coalesce, forming large, scaling patches or plaques with serous crusts and vesicles present at the periphery of the lesions. If the presence of the vesicles is not appreciated in these larger lesions, the location and appearance of the plaques can mimic psoriasis (Fig. 10.3). Other presentations can include urticarial plaques, bullous lesions, and larger erosions (Fig. 10.4). Occasionally, the presentation can be nonspecific, with only faint erythema and minimal scaling present on clinical examination (Fig. 10.5).
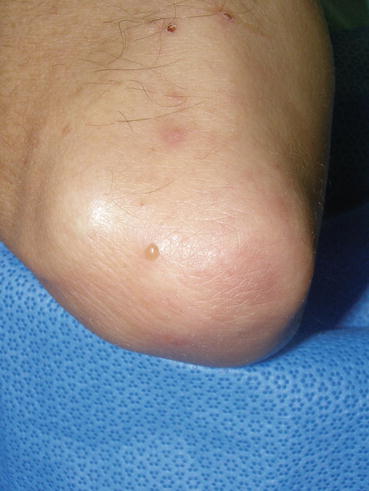
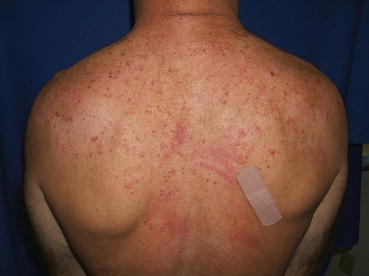
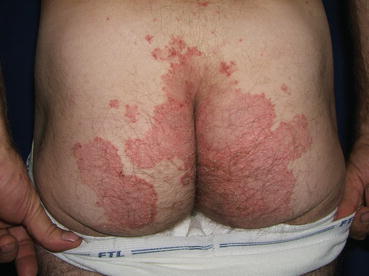
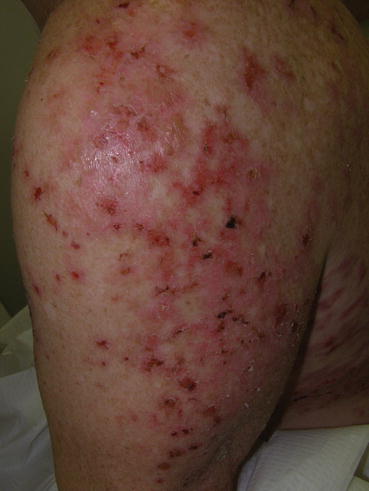
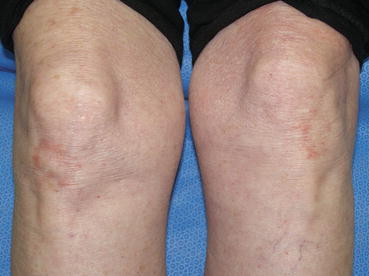

Fig. 10.1
Excoriations, postinflammatory erythema, and a vesicle on the right elbow

Fig. 10.2
Widespread excoriations with dermatographism on the upper back

Fig. 10.3
Erythematous scaling patches with peripheral microvesicles, excoriations, and erosions

Fig. 10.4
Larger erosions, hemorrhagic crusting, postinflammatory erythema, and one larger bulla on the lateral upper arm

Fig. 10.5
Faint erythema with minimal scaling on bilateral knees
An unusual presentation of DH is that of isolated digital or palmar petechiae, purpura, and microvesicles. This is a more common presentation in children [27], but adult cases have been described as well [28]. As the digital lesions can be the only manifestation of disease in these patients, a high degree of clinical suspicion is required.
Diagnosis
There are several diagnostic procedures that can be considered in making a diagnosis of DH, starting with a skin biopsy. Tissue can be taken from a vesicle or excoriated papule and sent for standard H&E analysis, with an additional biopsy from normal peri-lesional skin to be sent for immunofluorescence studies. Classic H&E findings in DH include a subepidermal bullae with sterile microabscesses present within one or more dermal papillae [29], although nonspecific histologic findings may be present in up to 22–37 % of cases [18, 30]. A much more sensitive test is that of direct immunofluorescence [DIF] of perilesional normal skin, which will demonstrate granular deposition of IgA in the dermal papillae in 97 % of cases [18]. Rare cases have been reported in the literature in which both the histology and DIF studies of patients with DH were negative [31]. In cases without classic H&E and immunofluorescence findings, the presence of circulating IgA anti-tissue transglutaminase antibodies and/or IgG/IgA anti-gliadin antibodies, in association with a pruritic eruption of erythematous papules and plaques, may raise the possibility of an atypical form of DH, and patients may respond to DH therapy. Serum testing for gliadin or transglutaminase antibodies is not completely sensitive, and the widely available IgA anti-tissue transglutaminase test will be positive in approximately 70 % of patients [18, 25]. Tests which can measure IgA anti-epidermal transglutaminase are more sensitive [95 %] [25], but are not widely commercially available.
Therapy
Medical Therapy
Dapsone is the only FDA approved medication for the treatment of DH. Initial doses are usually 50–100 mg a day, with adjustments as needed based on patient tolerance and response to therapy. Up to 300 mg a day may be required in some cases. Improvement in pruritus is noted almost immediately, while the resolution of the skin lesions may take several more days. Despite resolution of the skin lesions, immunoreactants are still present in patients taking dapsone who continue to consume a normal, gluten-containing diet [32], and the intestinal abnormalities that can be present are not influenced by dapsone therapy. In general, dapsone is tolerated well, although patients must be monitored on a regular basis. A glucose 6-phosphate dehydrogenase level should be checked prior to initiation of systemic dapsone therapy, and weekly CBC should be performed for the first month due to the possibility of agranulocytosis. A slight hemolytic anemia and methemoglobinemia, usually not significant enough to cause any symptoms, is expected in most patients. A hypersensitivity reaction can be seen rarely, and can occur up to 8 weeks following initiation of therapy. Long term side effects are also rare but include a peripheral neuropathy, most frequently a small muscle motor neuropathy, although sensory neuropathy has also been reported [33]. The mechanism of action of dapsone in the treatment of DH is largely unknown.
In patients that cannot tolerate dapsone, sulfasalazine has been reported as an effective therapy for DH [34], but the response in patients overall is less consistent when compared with dapsone. Isolated case reports describe the effectiveness of colchicine [35], and tetracycline and nicotinamide [36]. These options could be considered in patients who have documented allergies to sulfonamide medications.
Dietary Therapy
A strict gluten free diet will allow most patients to discontinue any medical therapy needed to control the skin lesions [19, 37]. Fry demonstrated that 80 % of DH patients that adhered to a gluten free diet were able to decrease the dose of dapsone required to control their skin disease, with some patients being able to discontinue the dapsone dose completely [37]. No patient was able to reduce the dapsone dose prior to 5 months on the gluten-free diet, and the time that it took to discontinue dapsone varied from 8 to 48 months [37]. Hence it is important to counsel patients that the response to the gluten-free diet is not immediate. Because of the severity of the pruritus and the time that it takes to respond to a gluten free diet, almost all DH patients are initially started on dapsone therapy, with initiation of the gluten free diet in order to decrease or discontinue the dapsone altogether. Iodine, either in dietary form or as a component of contrast agents, can cause a flare of skin lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree