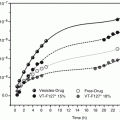
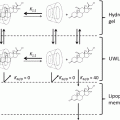
Fig. 15.1
Pathways of a compound from the topically applied product to the intended localization (Modified after Daniels and Knie (2007))
The ability of a drug to be liberated from the vehicle can be characterized by the ratio of the solubility of the active compound in the stratum corneum (C SC) to the solubility of the active compound in the vehicle (C V). This dependency can be described by the partition coefficient K SC/V according to Eq. 15.1 (Wiechers et al. 2004).
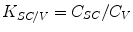
Thus, the drug amount which is liberated from the formulation into the stratum corneum can be enhanced either by increasing the solubility of the compound in the stratum corneum or by decreasing the solubility of the compound in the formulation (Wiechers et al. 2004).
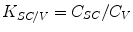
(15.1)
Topically applied compounds have to overcome the main skin barrier, the stratum corneum, which represents a complex mixture of various lipid and protein domains (Cross et al. 2001) in order to reach the underlying regions (Hadgraft and Pugh 1998). Thus, two processes, partition and diffusion, are important determinants for drug delivery into and through the skin (Wiechers et al. 2004), which in turn can be found in the flux of a compound according to Fick’s law of diffusion (Eq. 15.2) (Scheuplein 1976; Potts and Guy 1992):
![$$ J=\left(D\cdot {K}_{ow}\cdot \varDelta c\right)/x\kern0.24em \left[ mol\cdot {s}^{-1}\cdot c{m}^{-2}\right] $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_15_Chapter_Equ2.gif)
where J is solute flux, D is diffusion coefficient, K ow is octanol/water partition coefficient, Δc is drug concentration difference between the formulation and the deepest skin layer, and x is the length of the diffusion pathway of the active compound.
![$$ J=\left(D\cdot {K}_{ow}\cdot \varDelta c\right)/x\kern0.24em \left[ mol\cdot {s}^{-1}\cdot c{m}^{-2}\right] $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_15_Chapter_Equ2.gif)
(15.2)
Furthermore, the permeability coefficient K p is often used in order to simplify the permeation rate of an active compound through the skin (Eq. 15.3) (Hadgraft and Pugh 1998), since the actual diffusion pathway through the skin (x) is unknown. K p is furthermore the parameter used for statistical analysis of results of skin diffusion studies.
![$$ {K}_p=\left(D\cdot {K}_{ow}\right)/x\kern0.24em \left[ cm\cdot {s}^{-1}\right] $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_15_Chapter_Equ3.gif)
The number of transdermal applied compounds is still limited due to the lack of achieving therapeutic concentrations in the target tissue within the skin or in other body tissues after systemic circulation via blood or the lymphatic system (Bach and Lippold 1998), usually due to limitations in transdermal permeation caused by physicochemical drug characteristics (Magnusson et al. 2004). Especially the lipophilicity, the molecular weight (MW), and the melting point have to be taken into account in order to deliver a compound through the skin (Hadgraft and Pugh 1998; Magnusson et al. 2004; Nielsen et al. 2004). Potts and Guy (1992) demonstrated that the permeation of a topically applied drug in an aqueous solution can be estimated by two physicochemical characteristics, which are combined in Eq. 15.4 with MW as molecular weight of the active compound:
![$$ \log\;{k}_p=-6.3+0.71\; \log\;{K}_{ow}-0.0061\;MW\kern0.24em \left[ cm\cdot {s}^{-1}\right] $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_15_Chapter_Equ4.gif)
From Eq. 15.4 it can be concluded that small molecules (low MW) with adequate affinity to the horny layer (log K ow from −3 to +6) can be expected to exhibit a sufficient drug penetration (Bach and Lippold 1998; Potts and Guy 1992; Wiechers et al. 2004). Compounds with good affinity to both water and oil will show a good penetration behavior. Such compounds tend to exhibit a low melting point (Hadgraft 2004). According to Potts and Guy (1992), Eq. 15.4 can be used for substances with MW from 18 to >750 and log K ow from −3 to +6.
![$$ {K}_p=\left(D\cdot {K}_{ow}\right)/x\kern0.24em \left[ cm\cdot {s}^{-1}\right] $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_15_Chapter_Equ3.gif)
(15.3)
![$$ \log\;{k}_p=-6.3+0.71\; \log\;{K}_{ow}-0.0061\;MW\kern0.24em \left[ cm\cdot {s}^{-1}\right] $$](http://plasticsurgerykey.com/wp-content/uploads/2017/07/A309277_1_En_15_Chapter_Equ4.gif)
(15.4)
Furthermore, the rate of diffusion depends on the thermodynamic activity of the drug in the absence of penetration enhancing substances (Bach and Lippold 1998). It is characterized by the ratio of the drug concentration in the vehicle (C V) to the maximum solubility in the vehicle (C S) according to Eq. 15.5, with α as thermodynamic activity, C V as concentration of the active compound in the vehicle, and C S as saturation concentration of the active compound in the vehicle (Bach and Lippold 1998):
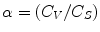
In this process the thermodynamic activity rises with the concentration of the compound up to the maximum concentration (saturation) in the vehicle (Hadgraft 2004). Thus, solutions show a decline of activity with the duration of incubation, while suspensions (having drug concentrations above their solubility) exhibit a constant activity (Bach and Lippold 1998).
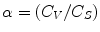
(15.5)
Commercially available topical formulations present a complex mixture of several classes of substances (Wiechers et al. 2004). The effect of all added substances in the formulation and the thermodynamic activity of the active compound have to be considered when transdermal and dermal drug delivery is predicted (Bach and Lippold 1998).
According to that, not only the properties of the active compound but also the vehicle influence the diffusion of the active compound through the skin. Vehicle ingredients can interact with the skin or with the active compound, so that its diffusion is enhanced or decreased.
15.2 Vehicle Composition
Although there is diverse nature of ingredients contained in vehicles used to deliver drugs into/through the skin (Wiechers et al. 2004), the following classification of vehicle components has been published by Daniels and Knie (2007) with five major classes.
15.2.1 Bases
Bases for topical products, representing the main ingredient of the product, can be either of hydrophilic nature like water or ethanol or they show hydrophobic characteristics (e.g., petrolatum, triglycerides) (Daniels and Knie 2007). The choice of the base depends on various factors, all of which have to be considered in the development of the final product. The base should exhibit no irritating or sensitizing potential to the skin, the active compound has to be delivered in adequate amounts to the site of action, and the shelf life of the product has to be long enough (International Pharmacopoeia 2014 ). Furthermore, a desired consistency has to be created (e.g., hydrophobic lipid bases can exhibit a special spreadable behavior over the skin without a greasy feeling) (Daniels and Knie 2007).
15.2.2 Gelling Agents
In order to enhance the viscosity of the product, which in turn improves storage and sensory attributes, macromolecules are added to form three-dimensional scaffolds. Popular thickeners are carbomer, hydroxypropyl cellulose, and carmellose sodium (Daniels and Knie 2007).
15.2.3 Preservatives (Antimicrobial Agents)
Preservatives against bacterial growth are necessary in order to enhance the shelf life of a product and for consumer protection against a contaminated product. Commonly used substances with properties of preservatives are ethanol or isopropanol, which have to be used in sufficient concentrations that means in concentrations about 20 %, which in turn are high enough to influence the penetration profile of a drug, as well. Furthermore, phenoxyethanol, benzoic acid, and sorbic acid are used in lower concentrations (0.1–0.2 % sorbic acid, 0.15–0.5 % benzoic acid, 0.5–1 % phenoxyethanol) (Daniels and Knie 2007). According to the International Pharmacopoeia (2014), antimicrobial agents are indicated unless the formulation itself has adequate preservative properties.
15.2.4 Antioxidants
These compounds are used in order to prevent oxidation reactions of lipids in exposition of light, air, and heat. Typical antioxidants are alpha-tocopherol in concentrations of 0.05–0.075 % and ascorbic acid esters in concentrations of 0.01–0.015 % (Daniels and Knie 2007).
15.2.5 Emulsifier
Emulsifiers added to the vehicle enhance the thermodynamic stability between hydrophilic and hydrophobic compounds. They are classified as anionic, cationic, amphoteric, and nonionic surfactants and accumulate between a lipid-rich phase and a hydrophilic phase (Daniels and Knie 2007). Table 15.1 lists some emulsifiers according to Daniels and Knie (2007).
Table 15.1
Classification of emulsifiers used in transdermal therapeutic systems according to Daniels and Knie (2007)
Compound classification | Examples |
|---|---|
Anionic | Sodium stearate |
Sodium dodecyl sulfate | |
Cationic | Benzalkonium bromide |
Cetylpyridinium chloride | |
Amphoteric | Phosphatidylcholine |
Betaine monohydrate | |
Nonionic | Glycerol monostearate |
Polysorbate 20 (Tween® 20) |
15.3 Formulation Types
According to the US Food and Drug Administration (FDA) (2013), topical dosage forms (Table 15.2) can be divided into semisolid and liquid formulations, which are characterized by their physicochemical nature. Especially the localization of the topical treatment and the severity of the disorder determine the kind of formulation, which is characterized by the content of oil and water (Daniels and Knie 2007). Ointments, e.g., are traditionally preferred by clinicians for topical use on hairless skin or areas with short hair, while creams are preferentially applied onto genial or flexural areas (Huang et al. 2005). Moreover, skin conditions have to be considered not only in healthy skin, but also in diseased skin. Acute or subacute diseases are often treated with formulations based on liquid bases (shake lotions or emulsions), while subchronic to chronic diseases are medicated with pastes, rich ointments, or gels. In brief, a more acute disease requires a high water and a low oil content (Daniels and Knie 2007). Thus, creams are preferentially used for wet conditions and ointments for dry conditions.
Semisolid and solid dosage forms | Liquid dosage forms | Specialties |
|---|---|---|
Creams and ointments | Emulsion | Microemulsion |
Gel | Lotion | Nanoemulsion |
Paste | Shampoo | Patch |
Powder | Solution | |
Spray | ||
Suspension |
15.3.1 Semisolid Formulations
A semisolid preparation exhibits no pourable behavior and does not conform to its storage container at room temperature. It does not flow, but exhibits plastic flow behavior. Low shear stress does not induce a flow of semisolid preparations. Semisolid dosage forms for topical treatment can be classified into creams and ointments, gels, pastes, and powders (International Pharmacopoeia 2014; FDA 2013).
15.3.1.1 Cream and Ointment
Creams and ointments are generally used for topical application and are semisolid preparations, in which solid or liquid substances are dispersed (Pharmacopoeia Europaea 2011). The vehicle contains >20 % water and volatiles and/or <50 % hydrocarbons, polyols, or waxes (FDA 2013). The consistency and rheological characteristics of creams depend on the emulsion type (oil in water or water in oil) and on the physicochemical properties of the solids used (International Pharmacopoeia 2014). Ointments are preparations which are “immiscible, miscible, or emulsifiable with skin secretions.” Therefore, bases of hydrophilic, hydrophobic, or water-emulsifying nature are used (International Pharmacopoeia 2014).
15.3.1.2 Gel
A gel is a clear preparation comprising a liquid phase (solution or colloidal dispersion) within a three-dimensional polymeric matrix caused by the addition of a gelling agent. It may contain suspended particles (International Pharmacopoeia 2014; FDA 2013). Gels can be divided into hydrophobic and hydrophilic gels.
15.3.1.3 Paste
15.3.1.4 Powder
Powders represent a mixture of drugs and/or chemicals, which are of dry and finely divided nature (FDA 2013).
15.3.2 Liquid Formulations
A liquid preparation is pourable and conforms to its storage container at room temperature. It flows and shows pseudoplastic flow and Newtonian behavior. The FDA (2013) distinguishes between emulsions, lotions, shampoos, solutions, sprays, and suspensions for external skin treatment.
15.3.2.1 Emulsion
Emulsions are characterized by at least two liquids that are immiscible, so that one component is dispersed in droplets within the other component. Thus, emulsions are special two-phase dosage forms. Stability is given by the addition of at least one emulsifier (FDA 2013).
15.3.2.2 Lotion
This dosage form is generally intended for topical application. A lotion represents an emulsion in a liquid dosage form (FDA 2013).
15.3.2.3 Shampoo
15.3.2.4 Solution
This dosage form is clear and homogenous. It may contain more than one chemical dissolved in one solvent or a mixture of miscible solvents (FDA 2013).
15.3.2.5 Spray
A spray is characterized by a liquid, which is divided into small components by a jet of air or steam (FDA 2013).
15.3.2.6 Suspension
This dosage form contains a liquid vehicle as the basis, in which solid particles are dispersed in (FDA 2013).
15.3.3 Special Formulation Types
Beside the conventional dosage forms, several new formulation types have been developed. Such a new preparation is a patch, which represents a sophisticated drug delivery system with the intent to release the active compound in such a manner that the dosing frequency can be reduced in comparison to conventional dosage forms (FDA 2013). Patches are commercially available for a constant delivery of various substances like ethinyl estradiol (Sachdeva et al. 2013), fentanyl (Lehmann and Zech 1992), lignocaine (Kwon et al. 2012), nicotine (Benowitz et al. 1991), nitroglycerol (Minghetti et al. 2001), rivastigmine (Gauthier et al. 2013), rotigotine (Stiasny-Kolster et al. 2013), scopolamine (Gil et al. 2012), testosterone (James 1995), and others. The main advantages of patches are sustained and constant plasma levels and the bypass of the first-pass effect in the liver (Kapil et al. 2012). Due to drug storage within the patch, transdermal patches can be classified into three types: (1) drug directly dispersed in adhesive polymer, (2) drug reservoir between a rate controlling membrane and a backing membrane, and (3) drug reservoir within the center of a peripheral adhesive ring around the edges (Sachdeva et al. 2013).
Furthermore, special liquid preparations have been generated in the last decades, such as nanoemulsions (Uner et al. 2005; Alves et al. 2007; Baboota et al. 2007), or microemulsions (Kemken et al. 1991; Boltri et al. 1994; Pattarino et al. 1994), etc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree