Fig. 17.1
A dentist with allergic contact dermatitis on his fingertips from 2-hydroxyethyl methacrylate (2-HEMA) in a bonding product. The dentist never wore gloves
In dental patients it is the mouth that is mostly affected if a contact allergy to a dental material is prevailing. The clinical picture is allergic contact stomatitis/gingivitis or cheilitis. In patients with clinical symptoms, the contact allergy frequency to denture base materials was 28 % [5, 6]. Contact allergy to acrylics is however uncommonly reported. It is delayed hypersensitivity to metals, cosmetics, food additives, flavors, and acrylates that dominates [1]. Clinical manifestations of gingivostomatitis are variable and include painful burning sensations in the mouth, local irritation, erythema, erosions, ulcerations, white plaques, mucosal swelling, sore mouth, and tingling. Clinical signs are often less pronounced than subjective symptoms. Allergic stomatitis is also rare [7]. Acrylics and metals such as mercury, gold, palladium, and manganese have caused stomatitis. Facial eruptions and systemic reactions can be seen. Diffuse erythema-like prosthesis stomatitis with stinging is seldom due to contact allergy but most often caused by Candida albicans in combination with an ill-fitting denture.
17.2 What Chemicals Should Be Tested When Suspecting Dental Materials?
17.2.1 Methacrylates and Acrylates
In particular methacrylates have been identified as major occupational contact sensitizers, both in dentists, dental nurses, and dental technicians. Three groups of acrylics are important in dentistry: (a) monofunctional methacrylates such as methylmethacrylate (MMA) and 2-hydroxyethylmethacrylate (2-HEMA), the latter common in bonding products, both MMA and 2-HEMA are semi-volatile; (b) multifunctional methacrylates such as ethyleneglycol dimethacrylate (EGDMA), triethyleneglycol dimethacrylate (TREGDMA), and triethyleneglycol diacrylate (TREGDA); and (c) acrylated and methacrylated prepolymers such as 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]-propane (bis-GMA) and urethane dimethacrylate (UEDMA), the former in dentin bonding products and both present in dental filling materials [8].
Dental composite resins (DCRs) are the filling material in white plastic fillings, and the most commonly used dental composite resin (DCR) is bis-GMA. This substance can be manufactured by an addition-reaction between diglycidyl ether of bisphenol A (DGEBA) resin and methacrylic acid. Hence, bis-GMA can be classified as a dimethacrylated epoxy, even if it lacks a reactive epoxy group [8]. DCRs may as a result contain DGEBA resin as an impurity. Therefore, a person sensitized to DGEBA resin may react to bis-GMA or vice versa, especially if that individual has a very strong hypersensitivity to DGEBA resin and/or bis-GMA. This can be elucidated if the patient is patch tested to these two preparations in serial dilutions and reacts to very dilute concentrations [4].
Dentin bonding agents are plastics without fillers, called resins. They are used as an adhesive to make the white plastic filling stick to the cavity of the tooth. After etching the surface to be treated with 37 % phosphoric acid, the dentin is covered with the bonding agent, which is pressed out into the cavity with pressurized air. Polymerization is then accomplished by blue visible light, and subsequently the DCR is applied to the cavity of the tooth in layers and cured. Curing is performed either with chemicals or with the same light as above. 2-HEMA is most often present in bonding systems as it is water soluble and hence does not damage the pulp, but bis-GMA, TREGDMA, and UEDMA can also be present. Because bis-GMA may be used in dentin bonding agents, DGEBA resin may also be present as an impurity.
17.2.2 Metals
There are few metals that seem to be important in relation to dental patients and their signs and symptoms from the oral mucosa and lips. These metals are mercury, gold, nickel, palladium, and titanium. For the dental profession these metals do not pose a risk in the occupational setting.
17.2.2.1 Mercury
In many countries mercury in amalgams has been replaced by acrylics due to environmental risks and the public’s opinion on worry of toxic effects. In Sweden it is prohibited to be used in dental practice since 2009. However, many people still have amalgams intraorally, and why this substance must be patch tested in dental patients. A Swedish study reported 9.3 % contact allergy to mercury in 1364 dental patients during a period of 11 years. The most common reasons for referral from the dentists were oral lichenoid lesions (39 %) and inflammation in the oral mucosa (16 %), whereas the most common subjective symptoms were burning sensation in the oral mucosa (13 %) [9]. Over 30 % of the mercury contact allergies would have been missed had not a day 7 reading been performed. In a recent study of 134 patch-tested dental patients, mercury allergy was noted in 9.9 %. Amalgam fillings may cause lichenoid lesions in the mouth as a contact reaction with or without contact allergy.
17.2.2.2 Gold
Contact allergy to gold is very common in dental patients, even in the oral absence of signs of contact allergy. A frequency around 25 % has been reported [10]. Studies have shown that there is a statistically significant correlation between dental gold and gold allergy. There is also a quantitative relationship between contact allergy to gold and the amount of gold areas in the oral cavity. A patient allergic to gold should not have new gold restorations fitted in their mouth, but removal of gold restorations in an allergic patient without apparent signs of contact allergy should not be done automatically. Metallic gold in crowns and other restorations has caused allergic contact stomatitis and gingivitis [11] (Fig. 17.2).
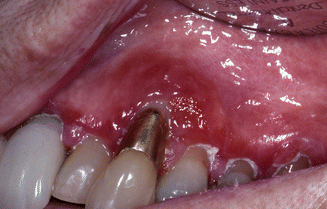

Fig. 17.2
A patient with gingivitis due to gold allergy. The patient has a metal ceramic crown containing gold
17.2.2.3 Nickel
Even if nickel allergy is common in the general population, it does not give any major problems when it comes to dental materials, as nickel is not used in permanent dental materials such as casting alloys in Sweden. However, in other countries, nickel has been used in such alloys without any major problems even in nickel-allergic individuals. As nickel and cobalt often are present in the same alloy, it is difficult to separate the two. Therefore nickel alloys often contain small amounts of cobalt (less than 1 %) and vice versa. If more than 0.1 % nickel is present in an alloy, it must be declared, but alloys containing less than 0.1 % nickel are considered nickel-free. In orthodontic alloys that are meant to be used only temporary, such as used in wires and brackets, stainless steel containing 18 % chromium and 8 % nickel is mostly used, but one may also use nickel-titanium thread, containing 54 % nickel and 46 % titanium. Orthodontic treatment with nickel-containing material in the mouth prior to ear piercing (nickel sensitization) does not seem to sensitize but rather give tolerance, and adverse reactions in nickel-allergic patients with orthodontic appliances containing nickel are uncommon [12].
17.2.2.4 Palladium
Palladium is one of the platinum group metals in the periodic table of elements. It is also resistant to corrosion. Palladium is a very common component of dental casting alloys of all types, e.g., together with dental gold, silver, zinc, and copper [1], in dental plates and as a catalyst in white gold. However, the risk of using palladium in dental casting alloys appears to be extremely low due to the low dissolution rate of the palladium ions from these alloys [13]. This can be interpreted such that even allergic patients tolerate these alloys. Thirty percent of those allergic to nickel react to palladium when patch tested, especially those with a strong contact allergy to nickel. Cross-reactivity between the two metals has been proposed and also shown in a scientific study when nickel was administered systemically [14]. The clinical significance of allergic reactions caused by palladium remains unclear, and only a few cases on contact allergy and allergic symptoms from palladium have been published.
17.2.2.5 Titanium
Dental implants based on titanium have been used since decades. Titanium allergy among dental patients is considered more or less nonexisting, even if some reports indicate that titanium may act as an allergen [15]. A retrospective study on dental patients tested to three titanium preparations (calcium titanate 10.0 % pet, elemental titanium as powder 50 % pet, titanium nitride 5.0 % pet) during 11 years and titanium oxalate 5.0 % pet for 1 year revealed 1 patient out of 1373 to react to calcium titanate on day 7 and not on day 3. There were 31 doubtful reactions in total. The authors concluded that titanium does not seem to sensitize dental patients and that it can be recommended for dental implants and frameworks for removable partial dentures [16].
17.3 Indications for Patch Testing: Who Should Be Patch Tested and When?
In dental professionals those with evident or suspected occupational contact dermatitis or worsening of an endogenous dermatitis in dental work should at least be patch tested with the baseline series and a dental series to find contact allergies or to out-rule them.
For dental patients, there are three major indications for patch testing:
1.
When a patient has objective signs in the oral mucosa localized next to a dental restorative material and when the clinical picture is a lichenoid reaction or when there is a strong suspicion of contact allergy to a dental restorative material.
2.
When a patient has a history of dermatitis in the face or elsewhere on the body and when there is a temporal relation to some dental treatment.
3.
When a patient is going to have a major dental restorative treatment and there is a history of intolerance to dental materials that will be used to out-rule contact allergy.
4.
A relative indication is the burning mouth syndrome.
In the burning mouth syndrome, physical signs of mucosal disease are missing, and patch testing is usually negative [17, 18], even though one study showed that 6 of 22 to have contact allergy to acrylics [19]. Most patients are denture wearers and some have infection with Candida albicans. Some consider “psychological factors” to be the most important [1]. In denture wearers with previous allergic diseases and the burning mouth syndrome, a high incidence of allergic skin reactions to denture allergens, especially methacrylates and formaldehyde, has been reported [20].
17.4 Which Dental Materials Should Be Tested and How Should the Test Procedure Be Carried Out?
A dental patient should be patch tested to a commercial dental patch test series. To date only few companies supply such a series. Two examples are Chemotechnique Diagnostics, Vellinge, Sweden, and Trolab Hermal, Hamburg, Germany.
The Swedish Contact Dermatitis Research Group has suggested two different dental series, based on previous patch test data from 15 Swedish clinics, one for the investigation of dental patients and one for the dental personnel (Tables 17.1 and 17.2). Dental personnel with suspected contact dermatitis from dental materials should be patch tested with a dental series plus a baseline series, whereas in dental patients a dental series is sufficient. Sometimes it may be difficult to judge the clinical relevance between a positive test and the dental patient’s signs and symptoms. The connection is best judged by the patient’s dentist in cooperation with the dermatologist.
Table 17.1




The dental screening series for dental personnel as recommended by the Swedish Contact Dermatitis Research Group
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







