Fig. 2.1
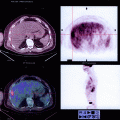
Early postoperative complication after construction of a J-shaped ileoanal pouch. (a) Schematic drawing and (b) radio-opaque contrast administration through the temporary ileostomy. Note the para-anastomotic extraluminal collection (arrows) due to leakage at the suture line. (c) An outpouring of radio-opaque contrast from the efferent loop (arrow) mimicking the presence of a sinus tract in the presacral region
The overall sensitivities reported by Thoeni et al. [7] for the detection of complications using different imaging modalities were as follows: 60 % with pouchography, 78 % with computed tomography (CT), and 79 % with In-labeled leukocyte scintigraphy [8]. According to these authors, fistulas were frequently missed with all three methods, whereas only CT correctly diagnosed all abscesses. Currently, magnetic resonance imaging is considered the method of choice and should be the initial test. If negative, a scintigram should then be obtained. In the absence of any adverse event, at 6–8 weeks after surgery, 200 ml of liquid barium is administered quickly from above through the ostomy (Fig. 2.2), resulting in full distension of the afferent segment of the reservoir so as to act as a “fluid overload” test. Radiographic imaging of the pouch provides a baseline for determination of reservoir capacity and acts as a broad prognostic indicator of future continence in patients who are continent during the radiologic “stress” conditions; these patients tend to display clinical continence after bowel reconstruction. Conversely, should there be only marginal continence with contrast media “stress” in the radiographic study, the time for stoma closure should be delayed until good control is obtained with a dedicated pelvic floor rehabilitation program.
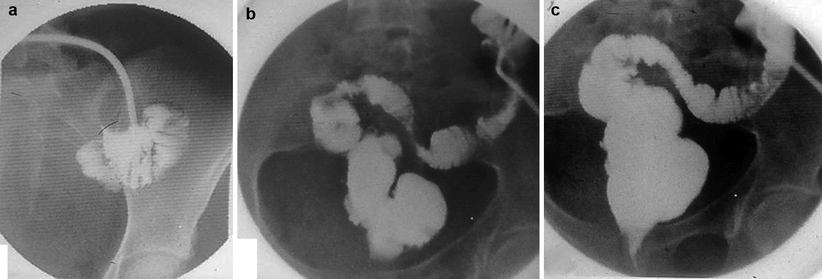

Fig. 2.2
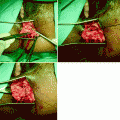
Fluid overload test performed before closure of the ileostomy and restoration of intestinal continuity: 200 ml of liquid barium administered through the ileostomy over 2 min. Images taken at 15 s (a), 60 s (b), and 90 s (c), respectively, to assess both the peristaltic activity and the capacity of the pouch without leakage
Although many factors are credited with determining future pouch function, including its volume, capacity, small-bowel motor activity, transit, gut hormone levels, sepsis, and bacterial overgrowth, to provide additional information about the emptying function of the reservoir, defecography [9, 10] usually is obtained 3 months after bowel reconstruction, in the lateral projection with the patient seated on a specially designed commode. For the examination, up to 200 ml of a semisolid barium sulphate suspension (Pronto Bario E, 70 % mass/volume, Bracco Spa, Milan, Italy) is administered transanally and the following features are noted:
1.
The overall geometrical configuration and size of the (neo)rectum at full distension, depending on the original pouch design.
2.
The position in the pelvis of the pouch, measured as the perpendicular distance of the ano-pouch junction from the pubococcygeal line, where the distal segment is defined, according to Pescatori et al. [11], as the tract of bowel between the pouch and the anal margin.
3.
The ano-pouch angle, defined as the angle between the luminal axis of the anal canal and the axis of the (neo)rectum or pouch obtained by drawing a line along the posterior wall of the distal rectum. This is preferred by the authors as opposed to the central axis of the rectal lumen, called the “centroid,” as described by Kmiot et al. [8], because we believe the latter measurement is excessively influenced by either the pouch design or the degree of filling. The angle is measured at rest and during squeezing, straining, and emptying.
4.
The pattern of pouch emptying (whether by a single movement or split), with the test being considered terminated only after the patient has been straining to evacuate as much of the introduced contrast material as possible for no less than 3 min.
5.
The amount of contrast retained, expressed as a fraction (one-third, two-thirds, or more) of the amount infused.
Defecographic findings associated with good functional results (e.g., stool frequency of no more than three to four movements per day and an absence of incontinence to gas or solid feces) include an anteroposterior diameter of the (neo)rectum not greater than 5–6 cm, no anal opacification or gaping at rest, mobility of the ano-pouch junction during squeezing and straining no less than 3 cm upward and no more than 3 cm downward, respectively; expulsion of the rectal content by no more than two to three movements within 60 s and a progressive decrease of the rectal diameter after filling by two-thirds during emptying, with no more than one-third barium retention at the end of the expulsion phase. On the other hand, major abnormal findings associated with poor functional results include an anal stricture, narrowing of the anastomotic ring, increased distance from the anastomotic ring to the anal verge due to progressive lengthening of the distal segment, and disproportionate enlargement of the pouch with difficult emptying and barium retention (Fig. 2.3).
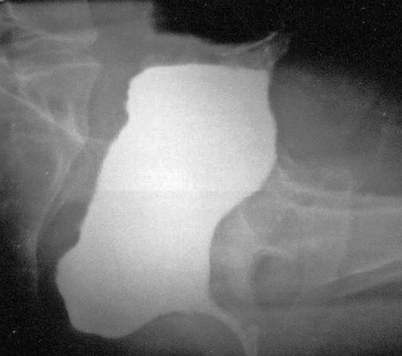

Fig. 2.3
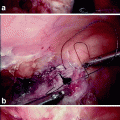
Lateral view of a W-shaped ileo-anal pouch after evacuation. Note the barium retention within the dilated pouch
Pouchitis is the complication reported to be best diagnosed by scintigraphy (sensitivity, 80 %), followed by CT scanning (71 %) and pouchography (53 %) [7]. Pouchitis is relatively common, with the incidence ranging from 9 to 34 % and an important impact on functional outcome causing increased stool frequency, pain during evacuation, urgency, anal irritation, and stool leakage. Possible etiological factors that have been reported to be responsible for pouchitis include abnormal pouch motility, leading to stasis, bacterial overgrowth, ischemia, or reperfusion injury, and occult Crohn’s disease [12, 13]. Histologic and endoscopic pouchitis is associated with leukocytosis, rheumatologic extraintestinal disease, disease initially proximal to the splenic flexure, age at diagnosis, and prior use of steroids. Intraoperative factors with greater risk of pouchitis include an S-pouch reconstruction, a multistage procedure, and perioperative transfusion, all of which are surrogate signs of operative complexity [14]. In a recent study by Lipman et al. [14] from the Cleveland Clinic, patients with pouchitis have worse outcomes than those without it, with more strictures, bowel obstructions, and fistulas and a lower quality of life. Histologic pouchitis that is found incidentally on biopsy and is asymptomatic does not seem to influence projected outcome.
Colo-Anal Anastomosis
Colo-anal anastomosis after tumor resection in the lower two thirds of the anus has rapidly gained acceptance worldwide despite some drawbacks, including excessive stool frequency and urgency, mainly caused by decreased compliance of the (neo)rectum. To improve functional outcome, the interposition of a J-shaped colonic segment just cranial to the anastomosis has been proposed by Lazorthes et al. [15] and Parc et al. [16] as an alternative to total proctectomy and straight colo-anal anastomosis. Although most authors claim the advantage of the J-shaped procedure over the straight coloanal anastomosis during the first 2 years after construction [17], no single diagnostic test has been proven particularly useful in predicting the superiority of one procedure over the other. Dynamic radiology (i.e., defecography) may help the clinician to obtain an objective assessment of the functional outcome [18, 19]. During the examination, the reconstructed anorectal junction is filled with a standard amount of semisolid barium sulphate suspension (200 ml of Pronto Bario E, 70 % mass/volume, Bracco Spa) with the patients lying on their left side on the table. The standard volume chosen corresponds to the sensation at which patients normally respond to the urge to defecate and is considered by the authors as more physiologic than the method in which the pouch is overdistended to the maximum volume tolerated. After withdrawing the probe, the table is tilted upright and the patient is positioned seated sideways on a specially designed commode (Bipot 125, Platinum, Giordanoshop, Naples, Italy). Intermittent fluoroscopy is used for both patient positioning and proper centering of the reconstructed bowel. Image acquisition is obtained directly from the intensifier using a video recording system that has playback and slow-motion facilities as well as a timer set at 100 per second.
The following phases are recorded with the patients seated in the lateral position: retrograde filling, upright at rest, squeeze, coughing, straining, and emptying. In addition, the ability to interrupt the barium stream on command, called the “stop test,” also is used and rated as maintained (0), reduced (−1), and lost (−2). Occasionally, anteroposterior and oblique views also are obtained. On sagittal images, the radiologist identifies and draws lines for computing distances and angles to register the following variables:
The maximum anteroposterior diameter of the (neo)rectum before and after evacuation
The pubococcygeal line extending from the inferior border of the pubic symphysis to the last point of the coccyx, representing the level of the pelvic floor
The distance from the anorectal junction on images obtained when the patient is at rest, during squeeze, at maximal straining, and during emptying
The colo-anal angle, defined as the angle between the luminal axis of the anal canal and the axis of the (neo)rectum or pouch, obtained by drawing a line along the projected posterior wall of the distal rectum.
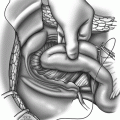
To estimate the pouch-anal angle, common difficulties encountered by the radiologist include a sigmoid loop–like appearance of the rectal ampulla, an asymmetric form of the distal rectum, an indistinct outline of the posterior rectal wall, and a variable impression of the puborectalis sling. Any impairment of pouch emptying caused by an anal stricture and/or abnormal angulation, lengthening of the segment distal to the anastomotic line (Fig. 2.4), as well as involuntary loss of contrast through the anus during the examination are noted.
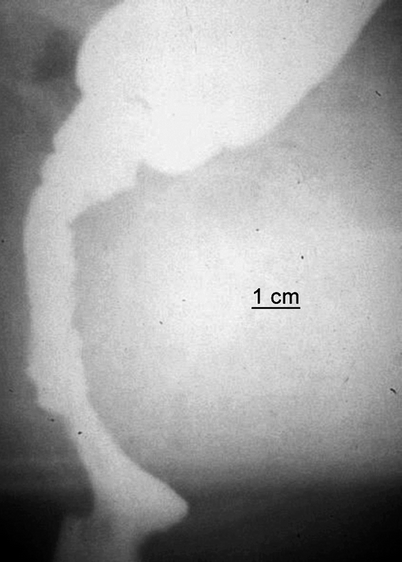

Fig. 2.4
Straight colo-anal anastomosis after rectal tumor resection. Postoperative defecography performed 1 year after surgery in a 54-year-old man with both difficult emptying and episodes of fecal incontinence. The segment distal to the suture line has become >7 cm in length and inert
Stapled Transanal Rectal Resection (STARR)
The STARR operation, first described by Longo [20] in the late 1990s as an alternative to traditional surgical techniques for the treatment of prolapsed hemorrhoids, subsequently was adopted for obstructed defecation syndrome (ODS) secondary to internal rectal intussusception, anterior rectocele, and rectal mucosal redundancy (rectal internal mucosal prolapse) after failed first-line medical therapy, rehabilitative therapy, or both. The procedure consists of two separate anterior and posterior rectotomies performed with the objective of restoring a more normal anatomy using two circular stapler devices (PPH-01, Ethicon Endo-Surgery, Cincinnati, OH) with modifications including the use of the STARR stapler and the trans-STARR staple device [21, 22]. The first is applied anteriorly to reduce the intussusception and rectocele, thus correcting the anterior rectal wall muscle defect, and the second is applied posteriorly to complete correction of the corresponding portion of intussusception. As such, the resection of 3–10 cm of full-thickness rectal wall is obtained, allowing coincident removal of both anatomical defects. In women, when the anterior rectal wall is resected, the posterior wall of the vagina is checked for potential damage to the rectovaginal septum and coincident small-bowel loops in an associated enterocele. The posterior rectal wall, however, cannot be similarly monitored because it lies directly on the puborectalis muscle, offering a potential risk for inadvertent entrapment of the muscle when closing and firing the circular stapler, which is a potential cause of severe postoperative proctalgia [23].
Two purse-string sutures are placed 2 and 5 cm above the anorectal ring, taking the mucosa, submucosa, and a small portion of the muscular wall. Instruments for bowel anastomosis were not originally designed to be hemostatic, requiring the use of reinforcement absorbable sutures to reduce bleeding at the staple line, although this need for reinforcement has been reduced with the introduction of the newer PPH-03 stapler for use during the procedure for prolapse and hemorrhoids/hemorrhoidopexy. Most frequently, the additional resection of two lateral bridges of residual mucosa, called “dog-ears” because separate anterior and posterior stapler firings are not performed equally around the circumference of the rectal wall, also is required. More recently, however, to improve the anatomical correction necessary for the best functional outcome, a new curved, cutting stapler device, called the Contour Transtar, has been developed; it allows for a more uniform, full-thickness, circumferential resection and a greater volume of tissue to be removed under the surgeon’s direct vision. Despite this, there are still significant complications reported, including rectovaginal fistula, incontinence, and anastomotic dehiscence [24], the assessment of which may require specialized radiology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree