Fig. 29.1
Electron microscopy of a malignant T cell in a patient with Sézary syndrome with characteristic prominent polychromatic cerebriform nucleus. Scale bar 2 μm
From an immunological perspective, patients with MF and SS have clinical abnormalities characteristic of Th2 mediated immunologic processes, including decreased antigen-specific T-cell responses, impaired cell mediated cytotoxicity, peripheral eosinophilia, and elevated levels of serum IgE [46]. Notably, the malignant T cells themselves are the main source of extreme excess of Th2 cytokines, and further enforce a global Th2 bias and suppress protective Th1 responses in CTCL individuals [27].
Additionally, increased IL-31 production in malignant T cells has recently been described to correlate with CTCL pruritus [45]; other important cytokines such as IFN-γ, IL-17, or TNF-α tend to be decreased, which likely accounts for the progressive impairment of cellular immunity and notorious susceptibility to infections in MF and SS individuals [33] (Fig. 29.2).
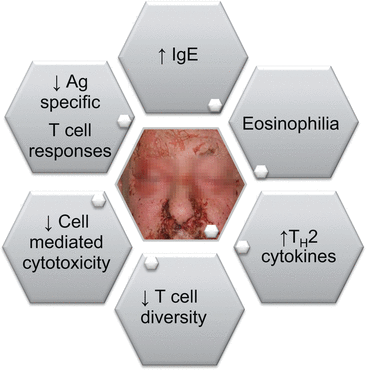

Fig. 29.2
Overview of immunological abnormalities in mycosis fungoides and Sézary syndrome patients
Activated dermal dendritic cells bridge innate and adaptive immune responses and are critical for tumor immune surveillance in the skin. Recently, Schwingshackl and colleagues analyzed the distribution and maturation status of different dendritic cell subsets in cutaneous lesions of MF and SS. Surprisingly, the numbers of dendritic cells are significantly increased within the skin infiltrate of both MF and CTCL; however, these dendritic cells are functionally largely immature. This phenomenon might be associated with induction of tumor tolerance or, on the contrary, may represent an on-going tumor immune surveillance [52]. Albeit critical observations on dendritic cells–T cells interactions in CTCL individuals are limited, activation status of the skin innate immune system might be a key factor for disease progression in CTCL [69, 70].
29.3 Cutaneous B-Cell Lymphomas
Primary cutaneous B-cell lymphomas (CBCL) are a heterogeneous group of lymphoproliferative disorders primarily arising in the skin that differ in biological behavior and prognosis [66]. The three most prevalent CBCL entities are part of the WHO classification of tumors of hematopoetic and lymphoid tissues as well as WHO-EORTC classification of cutaneous lymphomas: primary cutaneous marginal zone B-cell lymphoma (PCMZL), primary cutaneous follicle-center lymphoma (PCFCL), and primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL-LT), which account for >90 % of CBCL [56, 66]. In contrast to cutaneous T-cell lymphomas that can affect large skin areas, CBCL mainly form circumscribed skin lesions, such as singular or multiple papules and nodules. Different types seem to favor different anatomic sites, such as the upper back and arms in PCMZL, head and neck region for PCFCL and lower legs in PCDLBCL-LT [24].
Both PCFCL and PCLBCL-LT are large B-cell lymphoma with yet significantly different clinical courses and prognoses. The five-year survival rate of PCFCL is 95 % [53], and that of PCDLBCL-LT is ~50 % emphasizing its aggressiveness [26, 66]. Tumor cells in PCDLBCL-LT are mainly centroblasts with few admixed reactive elements. Tumor cells in PCFCL consist of follicle center cells with predominantly centrocytic differentiation. PCFCL can have a follicular, follicular and diffuse, or diffuse growth pattern. In contrast to nodal and secondary cutaneous follicular lymphomas, they do not express bcl-2 [8]. Gene expression profiling of PCFCL and PCDLBCL-LT revealed similarities to germinal center B-cell–like (GCB) and activated B-cell–like (ABC) nodal DLBCLs, respectively [29]. Likewise, immunohistochemical analysis could confirm overlaps between PCFCL and GCB [18, 71]. HGAL, B7-DC, MRC, CD130, and CXCR4, which are expressed in germinal center cells from normal tonsil were shown to be positive in PCFCL, but not in PCDLBCL-LT. On the other hand, most of PCLBCL-LT express MUM-1/IRF4 protein whereas PCFCL do not, which is associated with ABC nodal DLBCL [29]. Furthermore, PCDLBCL-LT also express cytoplasmic IgM with or without IgD in contrast to PCFCL. Array-based comparative genomic analysis also revealed differences in chromosomal alterations between PCFCL and PCDLBCL-LT [11].
PCMZL is a very indolent CBCL type with a 5-year disease-specific survival of almost 100 % [66]. Tumor cells of PCMZL consist of small B cells resembling marginal zone cells, lymphoplasmacytoid cells, and plasma cells. Characteristically, plasma cells can be observed particularly in the periphery of the tumor infiltrates. Neoplastic lymphocytes express B-cell markers (CD20, CD79a) and bcl-2, but are negative for CD5, CD10, and bcl-6. Infiltrating plasma cells typically display monoclonal expression of κ-and λ-Ig–light chains. The infiltrate may also include eosinophilic granulocytes, with moderate/marked tissue eosinophilia found almost only in Asian cases [58]. In the last WHO classification, PCMZL is assigned to the group of extranodal marginal zone lymphomas of the mucosa-associated lymphoid tissue (MALToma ). However, PCMZL is distinguishable from other extracutaneous MALTomas on various levels. Unlike other extranodal MALTomas, the translocations t(11;18) and t(14;18)(q32;q21) can be detected in only a small proportion of PCMZL. PCMZL express IgG, IgA, and IgE and thus differ from other extranodal MALTomas, which mostly express IgM. PCMZL with plasmacytic differentiation frequently express IgG4, whereas only 1 out of 120 noncutaneous marginal zone lymphomas expressed IgG4 [3]. Recently, PCMZL was recognized to encompass two subgroups [17, 64]. The more frequent one shows the presence of a class-switched immunoglobulin heavy chain and a distinct T-cell microenvironment with Th2-like cytokines opposite to Th1-like cytokines that characterize many extracutaneous MALTomas. In this subgroup, PCMZL are distinctive because the B cells frequently lack expression of CXCR3, a receptor for interferon -γ–induced chemokines, usually present on activated T cells. If PCMZL are nevertheless expressing CXCR3, they also express IgM and are in some cases associated with Borrelia burgdorferi infection [17].
An association with Borrelia burgdorferi infection and CBCL development has been reported in European cases [25, 38], which has certain parallels to Helicobacter pylori-associated MALTomas of the gastrointestinal tract [20]. Large geographic differences appear to exist, as an association with a Borrelia infection has not been observed in all European countries [48], the United States [68], and Asia [41]. On the other hand, Bahler et al. analyzed the immunoglobulin heavy chain variable genes from eight US cases of PCMZL and found the usage of V(H) genes and motifs that suggest antigen triggering as growth stimulator [1]. Furthermore, numerous CD3+ T cells and CD123+ plasmacytoid dendritic cells were described in PCMZL implying antigen presence [39]. Clusters of CD123+ plasmacytoid dendritic cells (PDC) are observed in all PCMZL and the majority of cutaneous B-cell pseudolymphomas and minority of PCFCL, whereas PCDLBCL-LT were negative. Although the role of PDC in PCMZL remains unclear, the high number of PDC may indicate an infectious cause or cross-reacting autoantigens as the primary trigger for PCMZL, as PDC migrate to nonlymphoid tissues in response to inflammatory or infectious stimuli. These findings indicate that a clonally restricted response to chronic antigenic stimulation could play a role in the pathogenesis of PCMZL [39].
Similar to cutaneous T-cell lymphomas (CTCL), a significantly increased number of mast cells is observed in CBCL lesions and those infiltrations are correlated with disease progression [51]. BCA-1 (CXCL-13) and its receptor CXCR5 are constantly expressed in CBCL, but not in healthy skin and CTCL. BCA-1 is also expressed in B-cell pseudolymphoma, and its expression pattern differs between CBCL and B-cell pseudolymphoma. BCA-1 expression is restricted to CD22+ B-cells in CBCL, whereas BCA-1 is expressed in CD35+ dendritic cells in B-cell pseudolymphoma [44]. Expression of programmed death-1 (PD-1) on T cells appears to have prognostic significance in some types of nodal B cell lymphomas [7]. Recently, PD-1 expression, restricted to tumor infiltrating T cells (TILs), was also shown in CBCL [43]. It appears that PCMZL and PCFCL harbor higher numbers of PD-1 positive TILs than PCDLBCL-LT, which in turn may also reflect better prognosis of the former. These data are in line with gene expression profiling studies on nodal follicle center lymphoma describing a more favorable clinical outcome in patients with a T-cell activation or subgroup signature [23].
In conclusion, CBCL represent a highly heterogeneous disease group that reflects morphologic and functional diversity of B-cells homing into the skin arising after malignant transformation.
29.4 Immunologic Therapeutic Interventions in Cutaneous Lymphomas
In the last decade, immense progress has been made in the biological therapy of primary cutaneous T- (CTCL) and B-cell (CBCL) lymphomas. It is, however, important to note that the majority of cutaneous lymphomas (CL) has an indolent nature and thus does not require aggressive treatment at the beginning. Studies have shown that early aggressive therapies do not improve the prognosis of patients with CTCL as compared with conservative treatment of topical therapies [31]. These and other data have led to a paradigm change in the therapy of CL, where cytotoxic therapies are not used as first-line treatment of indolent CL types. In early stages, the quality of life can be significantly impaired because of cosmetic concerns and itch, whereas advanced stages are often accompanied by immune dysfunctions leading to increased risk of infection and secondary malignancies. Hence, modern therapy algorithms consider the disease stage in order to achieve the best possible, sustainable, and life quality-preserving remission while reducing toxicity.
The simplest immunomodulatory skin-directed approach for CL lesions limited to the skin encompasses intermediate- and high-potency topical corticosteroids , with potency adapted to the infiltration of the lesion [2, 49]. Topically applied corticosteroids exert a multitude of inhibitory effects on both T and B cells which are well known [57] and result in efficient regression of skin lesions of different CL. Phototherapy is another effective skin-directed modality in the treatment of CTCL. Narrow-band ultraviolet B (UVB) light with a wavelength of 311 nm is becoming more popular over psoralen and UVA (PUVA ) therapy, as it requires no premedication with photosensitizing psoralen and has fewer side effects, especially in terms of photoaging and UV-associated carcinogenesis. Apart from induction of multiple immunomodulatory molecules, UVB light induces DNA damage with changes in the cell cycle leading to lymphocyte apoptosis [4]. PUVA, on the other hand, not only downregulates expression of skin-homing molecules of epidermotropic malignant T-cells; it is far more potent than UVB in inducing apoptosis of T-lymphocytes in the skin. Although both therapies can be employed with equal success, narrow-band UVB appears to be superior in cases with limited disease [2, 67]. A further development in the field of topical immunomodulators is imiquimod, a small molecule compound that activates Toll-like receptor (TLR) 7 and induces production of myriad pro-inflammatory cytokines [12]. By activating plasmacytoid dendritic cells that express TLR7 , imiquimod activates cytokine production, dominated by interferon (IFN) α, which counteract immune dysbalance in different CL types and promote antitumor immunity [61]. This effect could be potentiated by immunostimulatory activity of the isostearic acid, a major component of its cream vehicle [65].
Systemic immunomodulatory treatment is reserved for advanced stages or for therapy-resistant lymphoma lesions. For this purpose, various recombinant Th1-skewing cytokines, such as IFN-α and IFN-γ are currently routinely used either via intralesional or systemic route to treat these lymphomas [2, 12, 19, 67]. The goal is to reverse lymphoma-induced Th2-potentiated immunosuppression thereby stimulating antitumor immune effectors. To circumvent systemic toxicities, which can be a limiting factor in the treatment with IFNs, an adenovirus containing a human IFN-γ gene was constructed and tested for intralesional application in various CLs, showing overall good tolerability and higher response rates in CBCL than in CTCL [14, 16, 60]. Interestingly, patients who fail to respond to IFN-based treatments appear to have “IFN resistance,” that is, defects in the IFN signaling pathway [61, 62]. These defects can be successfully targeted by replicating measles virus that easily lyses lymphoma cells (i.e., oncolytic virus therapy ) as they are no longer able to mount an efficient antiviral response [13, 28]. TLR stimulation can also be achieved systemically by employing oligonucleotide CpG -7909 that acts as a TLR9 agonist and induces a similar plethora of cytokines as imiquimod. Promising results have been shown in CTCL patients [34], and further studies are on the way not only in T- but also in B-cell malignancies [40]. In advanced stages of CTCL with leukemic blood involvement, extracorporeal photopheresis (ECP) can be used alone or combined with IFN-α. ECP is a method where circulating mononuclear cells are separated by a leukapheresis-based technique, mixed with psoralen and reinfused to the patient. This therapy option, representing PUVA treatment of blood, is thought to induce apoptosis of circulating malignant lymphocytes and to stimulate antitumor response through release of tumor antigens and generation of dendritic cells from monocytes [67].
Development of monoclonal antibodies (mAbs) targeting surface molecules on T and B cells is a fast growing addition to the repertoire of immunotherapeutic options in CL. In CTCL, targeting CD4 by zanolimumab and CD52 by alemtuzumab shows variable success depending on the lymphoma type treated [2]. Denileukin–diftitox is a fusion protein, which consists of diphtheria toxin fragments conjugated to interleukin 2. After binding to interleukin-2–receptor this molecule is internalized, where it leads to inhibition of protein synthesis and apoptosis [21]. Its activity as a single agent or in combination with chemotherapy has been shown in CTCL patients who are CD25 positive [30]. Brentuximab–vendotin is a conjugate of an antibody against CD30 with methyl-auristatin E, a tubulin destabilizing agent, that can be selectively used for CD30+ CL [63].
In CBCL, expression of CD20 is mandatory for the treatment with rituximab, a human–mouse chimeric antibody against CD20. Rituximab can be used either via an intralesional or systemic route and has shown impressive responses in CBCL [19]. Anti-CD20 antibodies can also be conjugated with various radionuclides, such as yttrium (90Y-ibritumomab tiuxetan ) or iodine (131I-tositumomab ) and applied with or without chemotherapy in relapsed patients or patients with refractory disease [59]. Several additional antibodies targeting other molecules on B cells, such as CD22 (epratuzumab ), CD19 (Medi-551 ), and their toxin conjugates inotuzumab-ozogamicin (CD22) and SAR3419 (CD19) come from the field of nodal lymphomas and may be of interest in CBCL. Bispecific T-cell–engager molecules, also known as BiTEs, are another novelty and represent single-chain dual-specific antibodies that target both CD3 on the surface of T cells and a specific antigen on the surface of the malignant B cell in order to induce cell lysis. One BiTE with specificity to CD19, blinatumomab (MEDI-538), has demonstrated high clinical activity in B-cell leukemia and lymphoma patients [59].
Lastly, it should not be left unsaid that not only immunotherapeutics are engaged in immune modulation, but also other compounds used for CL, such as histone deacetylase inhibitors , may have profound immunomodulatory properties through regulation of innate and adaptive immune responses [42]. If all therapies fail, allogeneic stem cell transplantation may become the last resort, particularly in CTCL, inasmuch as autologous stem cell transplantation has yielded disappointing results. Immune interventions in CL have and are working well, emphasizing strong immunogenicity of these tumors and giving hope that the time will come where a therapeutic intervention will not only stabilize but be able to cure the disease.
References
1.
Bahler DW, Kim BK, Gao A, Swerdlow SH (2006) Analysis of immunoglobulin V genes suggests cutaneous marginal zone B-cell lymphomas recognise similar antigens. Br J Haematol 132(5):571–575. doi:10.1111/j.1365-2141.2005.05904.x CrossRefPubMed
2.
Belloni B, Johansen N, Glass LF, Dummer R (2012) Recent advances in the management of cutaneous lymphomas. Semin Oncol 39(2):150–162. doi:10.1053/j.seminoncol.2012.01.010 CrossRefPubMed
3.
Brenner I, Roth S, Puppe B, Wobser M, Rosenwald A, Geissinger E (2013) Primary cutaneous marginal zone lymphomas with plasmacytic differentiation show frequent IgG4 expression. Mod Pathol. doi:10.1038/modpathol.2013.106 PubMed
4.
Bulat V, Situm M, Dediol I, Ljubicić I, Bradić L (2011) The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Anthropol 35(Suppl 2):147–151
5.
Burg G, Kempf W, Haeffner AC, Nestle FO, Hess Schmid M, Doebbeling U et al (1997) Cutaneous lymphomas. Curr Probl Dermatol 9(5):137–204CrossRef
6.
Campbell JJ, Clark RA, Watanabe R, Kupper TS (2010) Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood 116(5):767–771. doi:10.1182/blood-2009-11-251926 CrossRefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








