Fig. 24.1
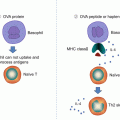
(a) The hapten-modified peptide is recognized and stimulates T cells. The hapten may also have the ability to activate the innate immune system. (b) The drug binds to the TCR and provides some initial signal. The signal is strengthened by additional interaction with HLA molecules. Drug binding and HLA interactions stimulate T cells as does a normal peptide/HLA complex
24.2.2 The p-i Concept
As an alternative to the hapten and pro-hapten concepts, the p-i concept proposes that a drug is able to stimulate T cells directly without forming a hapten, in an HLA-dependent manner [32]. This may occur if a drug that cannot form a covalent bond with a larger carrier interacts directly with T-cell receptors (TCR) or MHC molecules with sufficient affinity.
According to the hapten and pro-hapten concepts, drugs and other substances that are not chemically active and that are therefore incapable of coupling to a protein would not be antigens and could not induce hypersensitivity reactions. However, this hypothesis has been challenged by clinical and immunological evidence that cannot be explained by hapten or pro-hapten models [34].
Consistent with the p-i concept, chemically inert drugs, unable to bind covalently to peptides or proteins, can nevertheless activate certain T cells, if they fit with a sufficient affinity into some of the various T-cell receptors or MHC molecules (Fig. 24.1). Evidence for the p-i mechanism lies in observations in which fixed APC, which are unable to process antigens, are still able to activate specific T-cell clones. Instead of a drug (hapten) specific immune response, the p-i–stimulated T cells arise from previously primed effector and memory T cells. In vivo, p-i–activated T cells expand and subsequently infiltrate the skin and other organs. This threshold of T-cell activation might be further lowered by the massive immune stimulation that concomitantly occurs during generalized herpes, human immunodeficiency virus (HIV) infections, autoimmune diseases, or malignant tumors. Such immune processes are associated with high cytokine levels and increased expression of MHC and costimulatory molecules. Consequently, T cells more readily react to a minor signal, such as the binding of a drug to its TCR. This would explain the high occurrence of drug hypersensitivities in these diseases. On the other hand, in some cases, metabolites simultaneously react as well as the parent compound. This suggests that the hapten characteristic of a drug is required for p-i stimulation to occur.
24.2.3 Affects of HLA Polymorphism on Drug Antigen Peptide Presentation
In general, peptides associate with HLA molecules by inserting parts of their amino-acid residues into a set of six binding pockets in the HLA [23]. The structure of these pockets is highly allele-specific, thereby dictating peptide-binding preferences for each HLA molecule.
A recent paper showed that a drug can bind directly to some pocket of a specific HLA, but that the drug does not bind to a closely related HLA molecule [17]. These data suggest that HLA and the drug form a complex before the HLA molecules are loaded with peptides inside the cell, thereby altering the pool of self peptides that are bound to the HLA and displayed on the cell surface for T-cell recognition. This shift in the specific HLA-associated cell-surface–peptide display leads to the activation of different T cells. Indeed, activation of a wide range of CD8+ T cells occurs as the cellular basis of abacavir hypersensitivity reactions [7].
Part of the drug protrudes into the HLA molecule’s pocket, reducing the pocket’s size, which accounts for its preferential binding of smaller amino acids following drug exposure. The structures also revealed that the drug binds to the amino-acid residues that are unique to the HLA molecule, thereby explaining the drug’s allele specificity. This shift in the bound-peptide repertoire is a plausible explanation for drug-induced hypersensitivity [36], T cells that lack self-reactivity but have the potential to recognize foreign antigens.
Some self peptides are never encountered during T-cell development, but exposure can occur under pathological conditions [7]. When this occurs, a situation of “mistaken identity” can arise, in which self peptides are perceived as foreign by the immune system.
In complexes that consist of immunogenic HLA, the drug and peptide may be generated either via incorporation of the drug with peptides of the constitutive repertoire, or within the ER, such that the peptides are presented with an altered conformation, or the stabilization of “novel self” ligands may be absent from the constitutive repertoire but favored in the presence of the drug.
24.3 Cytotoxic Signals and Immune Molecules in SJS/TEN
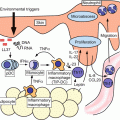
The phenomenon of antigen (causative drug) presentation seems to be shared by SJS/TEN and nonsevere adverse drug reactions. Indeed, several HLA haplotypes that have been reported to be associated with adverse drug reactions do correlate with severity [46]. With regard to histological findings, an observation of keratinocyte death differentiates SJS/TEN from nonsevere adverse drug reactions. Therefore keratinocyte death may be implicated in the SJS/TEN pathomechanism.
24.3.1 Fas–FasL Interaction
In 1998, French and colleagues reported that the activation of the Fas apoptosis receptor through the Fas ligand (FasL) is an initial important step in keratinocyte apoptosis in TEN [45]. They assumed that both Fas and FasL derived from keratinocytes, and that the FasL expressed by keratinocytes leads to the apoptosis of keratinocytes in TEN [45]. The present author previously showed that the levels of soluble FasL (sFasL) in SJS/TEN patient serum are elevated, that sFasL is secreted by causal-drug–stimulated peripheral blood mononuclear cells, and that TEN patient serum with high levels of sFasL induces apoptosis in cultured keratinocytes [1].
Before disease onset (Day -4~Day -2), seven samples were available, and we detected the high concentrations of sFasL in five out of seven cases (71.4 %). The elevated sFasL level declined rapidly within 5 days after disease onset. In all 32 patients with ODSR and in the 33 normal controls, no elevation of sFasL was detected. Other soluble factor concentrations showed no significant difference for SJS/TEN before disease onset versus for nonsevere adverse drug reactions [29]. Lan also reported a diagnostic role for sFasL secretion by peripheral blood mononuclear cells from patients who had recovered from SJS/TEN [20]. On the other hand, other groups reported that elevated levels of FasL were detected not only in TEN patients but also in the sera and lesional skin of patients with the maculopapular type of adverse drug reactions [41]. Drug-induced hypersensitivity syndrome (DIHS), another severe adverse drug reaction, also shows high serum levels of sFasL [44].
24.3.2 Perforin and Granzyme B
Perforin/granzyme B have been reported to play a key role in keratinocyte death in SJS/TEN [30]. Nassif et al. showed that the cytotoxic effect of TEN blister lymphocytes on keratinocytes could be attenuated by the inhibition of perforin/granzyme B expression, but not by the anti-Fas monoclonal antibody [30]. The activated CTLs and NK cells produce perforin, which can bind and punch a channel to the target cell membrane and promote the entrance of granzyme B into keratinocytes. Once the granzyme B enters into the target cells, it activates the caspase cascade and the succeeding apoptosis [22]. Levels of perforin, granzyme B, TNF-alpha, and FasL have been observed to relate to disease severity of drug hypersensitivity, from mild maculopapular rashes to severe TEN [35].
24.3.3 Granulysin
Granulysin is a cytotoxic molecule that is produced against virus-infected cells, tumor cells, transplant cells, bacteria, fungi, and parasites [18]. It plays an important role in the host defense against pathogens. The 15-kDa granulysin, a cationic cytolytic protein, is secreted extracellularly by CTLs and NK cells via a nongranule exocytotic pathway [19]. The expression level of granulysin rises upon T and NK cell activation. Granulysin has been reported as a serum marker for cell-mediated immunity. Chung and his colleagues reported that granulysin is strongly expressed by blister cells in skin lesions and plays a crucial role in the widespread keratinocyte apoptosis of SJS/TEN [10]. Granulysin has a direct cytotoxic effect on keratinocytes at concentrations detected in the blister fluids. The cytotoxic effect of SJS/TEN blister fluids on keratinocytes can be reduced by granulysin depletion. In addition, injections of granulysin into mouse skin were found to result in blistering and epidermal necrosis mimicking SJS/TEN [10]. They concluded that high levels of secretory granulysin in blistering skin lesions could explain the histopathology observed in SJS/TEN, in which infiltration of sparse dermal mononuclear cells results in extensive epidermal necrosis. In addition, the serum levels of granulysin were found to increase during the early stage of SJS/TEN, but not in patients with drug-induced MPE [2], suggesting granulysin as an early diagnostic marker of SJS/TEN. Indeed the immunochromatographic test for granulysin (with a procedure time of less than 15 min) showed positive results for 4 out of 5 patients with SJS/TEN but only 1 patient out of 24 with nonsevere adverse drug reactions [12]. The results correlate closely with those of ELISA. On the other hand, DIHS also shows high serum levels of granulysin [38].
24.3.4 Other Cytokines/Chemokines
To date, several reports have shown cytokines/chemokines as being involved in the immune reactions of SJS/TEN. These cytokines/chemokines were found to have elevated expression in skin lesions, blister fluids, blister cells, peripheral mononuclear cells, or plasma in SJS/TEN. These cytokines/chemokines include IFN-g, TNF-a, IL-2, IL-5, IL-6, IL-10, IL-12, IL-13, IL-15, IL-18, CCR3, CXCR3, CXCR4, and CCR10 [8]. These cytokines/chemokines may be responsible for the trafficking, proliferation, regulation, or activation of T cells and other leukocytes involved in SJS/TEN. In addition, the α-defensin gene was recently found to be expressed in PBMCs from patients with cutaneous adverse drug reactions [26]. α-Defensin expression was confirmed by intracellular flow cytometry in mononuclear cells from the patients, including monocytes, NK cells, and T cells from peripheral blood and blister fluid. α-Defensin levels were estimated by ELISA to be higher in blister fluid when compared to simultaneously drawn plasma samples.
24.4 Subtypes of T Lymphocytes
CD8+ lymphocytes are necessary to SJS/TEN pathogenesis. Reports have shown that CD4+ T cells are the predominant population that infiltrates into “maculopapular rash” skin lesions and that most drug-specific T cells are CD4+ T cells [15]. However, in severe cutaneous adverse drug reactions, CD8+ T cells were found to be the predominant population that infiltrated into the epidermis of skin lesions of SJS/TEN patients [10], and HLA-B*1502 was found to be associated with carbamazepine-induced SJS in all cases [9]. In addition, drug-specific CD8+ T cells were found to proliferate predominantly during the acute stages of SJS/TEN [14]. Although drug-specific CD4+ T cells are essential in drug-mediated immune reaction, CD8+ T cells are critical to the development of SJS/TEN.
If SJS/TEN is the ultimate adverse drug reaction along the spectrum of nonsevere adverse drug reaction, then regulation of immunological reaction could influence the severity of the reactions. For example, immunocompromised individuals, such as patents who have AIDS or malignancy, or who were treated with immunosuppresive therapy, tend to be prone to SJS/TEN [24, 40].
24.4.1 Regulatory T Cells
The regulatory T cell (Treg) maintains self-tolerance and suppresses immune responses. Treg has been reported to be involved in the pathogenesis of SJS/TEN [4, 42]. Treg function is profoundly impaired in TEN, even though the cells are present in normal frequency [42]. These functional defects in TEN are restored upon recovery. These findings indicate that transitory impairment in their function during the acute stage of TEN may relate to severe epidermal damage, whereas a gradual loss of their function after resolution of DIHS may increase the subsequent risk of autoimmune disease occurrence [42]. Indeed, in an animal model of TEN, Treg cells were found to prevent experimentally induced epidermal injury mimicking TEN [3, 4].
Recent reports have shown that CD4+Foxp3+ Treg exists in heterogeneous subpopulations divided by the level of CD45RA expression [25]. Human CD4+Foxp3+ Treg is divisible into three functionally and phenotypically distinct subsets: CD4+CD45RA+ Foxp3low resting Treg (rTreg), CD4+CD45RA−Foxp3high activated Treg (aTreg), and cytokine-secreting CD4+CD45RA−Foxp3low nonsuppressive T cells (non-Treg). rTreg and aTreg have potent immunosuppressive activity, whereas non-Treg lacks such activity and has the potential to secrete pro-inflammatory cytokines such as IFN-γ and IL-17 [25]. Furthermore, the relative frequencies of the three CD4+Foxp3+ Treg subpopulations differ with various disease conditions [31, 39, 48]: in active systemic lupus erythematosus, for example, the number of aTreg cells is lower and that of non-Treg cells is higher than those of healthy controls, enabling an autoimmune reaction [25]. SJS/TEN might also be considered susceptible to development under the imbalance of CD4+Foxp3+ Treg subpopulations. The relative frequencies of the three Treg subpopulations and cytokine-secreting activity are found to differ between SJS/TEN patients and healthy controls. These results indicate that the imbalance of Treg subpopulations is involved in the pathogenesis of SJS/TEN.
24.4.2 Th17 Cell
Th17 cells are a recently described effector CD4+ T-cell subset that produces IL-17 and IL-22 and that has been implicated in the pathogenesis of various autoimmune and allergic diseases [21]. The proportion of circulating IL-17–producing CD4+ T cells, but not CD8+ T cells, is significantly higher in patients with SJS/TEN than in patients with erythema multiforme, as well as in healthy subjects [43]. IL-17–producing CD4+ T cells in a CLA+CCR4+ subset with skin-homing properties are found in significantly higher proportion in this subset of patients with SJS/TEN [43]. The proportion of circulating Th17 cells decreases significantly after disease improvement. Collectively, these results suggest that skin-homing Th17 cells are involved in the pathogenesis of SJS/TEN. Th17 cells might be involved in inflammation and tissue damage in patients with SJS/TEN through regulation of the recruitment of neutrophils and other inflammatory leukocytes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree