Chapter 13 Cosmeceutical Metals
INTRODUCTION
Are certain topically applied metal ions simply innocuous treatments or do they provide real technical benefit? Their use goes back to the earliest recorded medical text (∼1500 BC), the Ebers papyrus of ancient Egypt. For example, calamine (a natural material containing zinc oxide) was described for treating many skin and eye ailments; green copper-based minerals (likely malachite) were used for burn wounds and itching. Many of these applications have withstood the ensuing 3500 years of history, providing a first clue of real technical merit. For example, zinc is still the first choice to soothe a crying baby’s bottom.
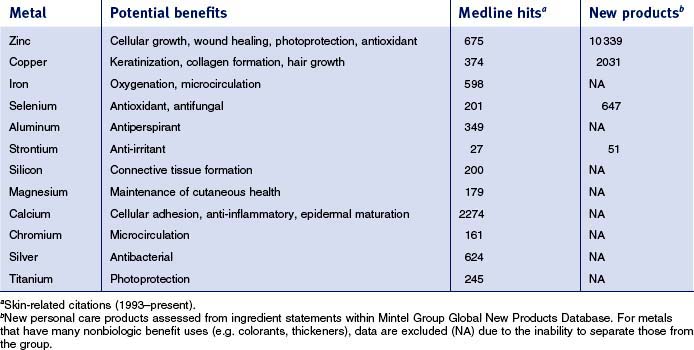
This review will focus specifically on five metals—zinc, copper, selenium, aluminum, and strontium—which are currently used in cosmeceuticals. Each metal will be covered sequentially, reviewing commonly used materials followed by clinical and scientific data supporting their use. There are many other metals that have found usage in cosmeceuticals (see Table 13.1). Searching the skin-related literature (Medline, 1993–present) demonstrates that there is substantial scientific activity exploring the technical basis for utilization of some of these metals. Also summarized in Table 13.1 for some of the metals is the introduction of new personal care products containing them; substantial commercial activity is evident.
ZINC IN COSMECEUTICAL PRODUCTS
• Materials
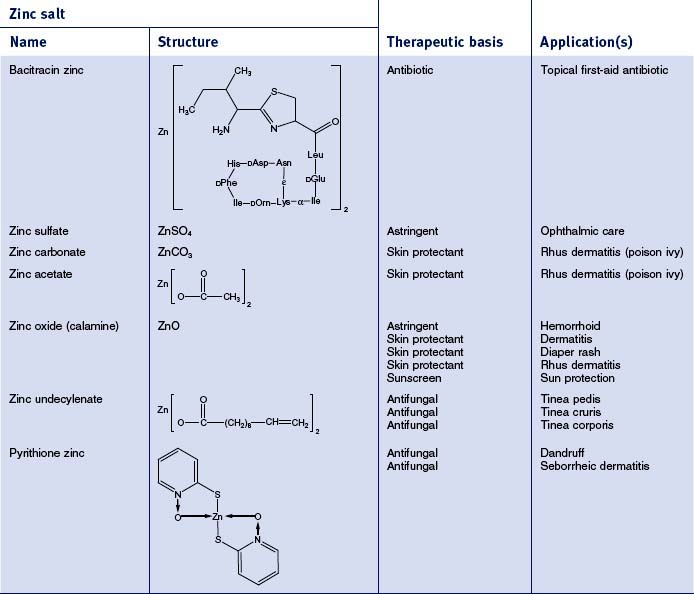
There are 55 different zinc-containing materials listed in the International Cosmetic Ingredient (INCI) Dictionary and Handbook (a tabulation of all materials used in cosmetic and personal care products). Of those, seven have been approved by the US Food and Drug Administration (FDA) for over-the-counter (OTC) usage as safe and effective for a range of benefits, including skin protection, antimicrobial activity, and astringency (Table 13.2). The skin protective benefits of these zinc materials find applications treating various inflammatory dermatitis conditions such as poison ivy and diaper rash. The wide range of zinc materials approved by the FDA provides a strong indication of the general utility of zinc as an effective treatment.
• Basis for use of zinc materials
CLINICAL PERSPECTIVE
Zinc has also recently been shown to improve skin elasticity, reducing the signs of aging skin.
SCIENTIFIC FOUNDATION
An average human contains 2.5 g of zinc and requires 15 mg/day to remain healthy (this is exceeded only by iron for trace elements). The vast majority of the zinc is present in metalloenzymes and proteins. This field was opened in 1940 with the discovery that carbonic anhydrase, a ubiquitous enzyme required for maintaining physiologic pH, was zinc-containing and that the zinc was required for catalytic activity. Since that time, over 300 enzymes requiring zinc for activity have been structurally characterized. Even more impressive are the thousands of zinc-containing proteins that require zinc for conferring a three-dimensional structure that allows them to regulate replication of DNA and transcription of RNA. These proteins form the class called ‘zinc fingers’ and regulate the fundamental biologic process of transcribing genetic information to functional proteins. At least 3% of all proteins encoded for by the human genome have zinc fingers; this has led Berg to coin the phrase ‘galvanization of biology’ to acknowledge the importance of this metal in human physiology.
While it is beyond the scope here to review many of the zinc-containing biomolecules (see Table 13.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree