Chapter 12 Cosmeceutical Botanicals: Part 2
INTRODUCTION
The explosive growth of the cosmeceutical industry has resulted in the introduction of many new ‘active’ ingredients. Most are derived from nature for unique marketing stories and to reduce the risk of federal oversight. About 100 different ingredients derived from plant sources are being incorporated into cosmeceutical and skin care products. It behooves all physicians and skincare providers recommending cosmeceuticals to have a working knowledge of botanicals so the best recommendations can be made to patients and clients.
 complete characterization of the huge number of active compounds in a single plant source
complete characterization of the huge number of active compounds in a single plant source
 documenting the activity and interaction of each of these compounds and their many metabolites
documenting the activity and interaction of each of these compounds and their many metabolites
 understanding the therapeutic synergy of these active components within the single plant and between multiple plants
understanding the therapeutic synergy of these active components within the single plant and between multiple plants
 discovering how the potential toxicity of specific compounds is modified by using an entire plant or an anatomic structure of the plant—for example, the castor bean is the source of ricin, one of the most poisonous compounds known to man, and azelaic acid, a nontoxic prescription dermatologic medicine.
discovering how the potential toxicity of specific compounds is modified by using an entire plant or an anatomic structure of the plant—for example, the castor bean is the source of ricin, one of the most poisonous compounds known to man, and azelaic acid, a nontoxic prescription dermatologic medicine.
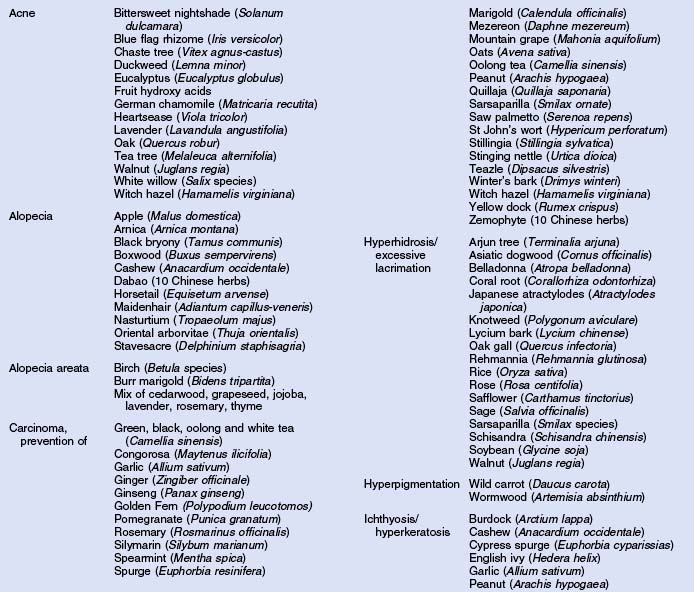
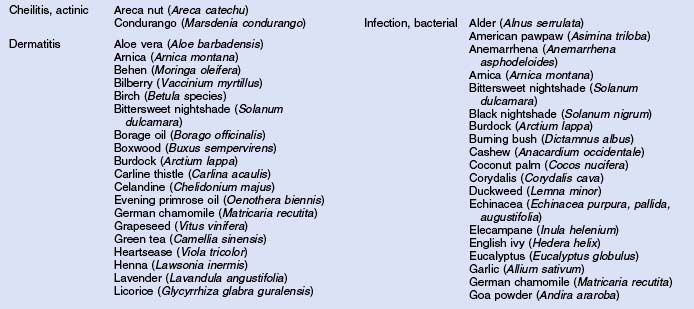
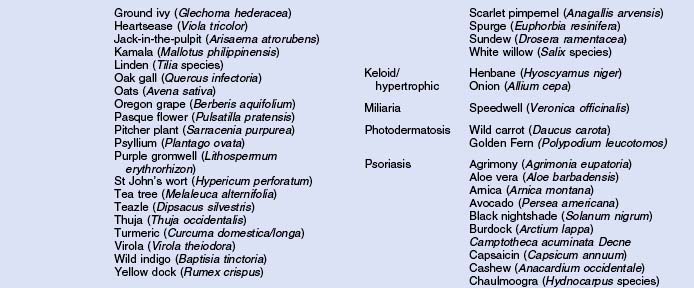
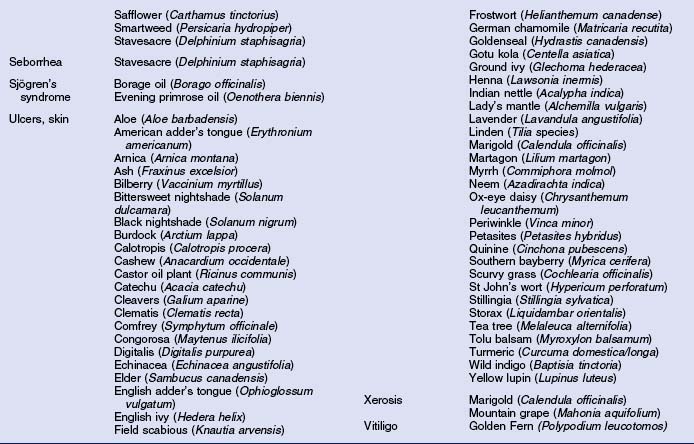
Herbs with mucocutaneous therapeutic indications as determined by Commission E and PhytoPharm are listed in Table 12.1. The first group of herbs discussed below consists of most of the top 12 largest selling herbs in the US based on dollar volume. Of all the herbs listed with mucocutaneous applications, only 27 have been supported by reliable double-blind or open label clinical trials as listed in Table 12.2. The second group consists of most of the 21 herbs formulated into products tested in blinded clinical trials for treatment of photoaging as listed in Table 12.3.
Table 12.2 Herbs with clinical trials for treatment of mucocutaneous diseases/conditions
Table 12.3 Herbs with clinical trials for photoaging therapy