Fig. 20.1
Representative image of neutrophil infiltration and T cells–DCs interaction in skin during elicitation phase. (a) Accumulation of neutrophils in dermis 24 h after elicitation (Green: neutrophils in Lysm-eGFP mice. Red: transferred CD4/CD8 T cells). (b) The interaction between T cells and dendritic cells in skin 24 h after elicitation (Green: Langerin positive cells in Langerin-GFP mice. Red: transferred CD4/CD8 T cells)
20.4.2 Antigen Specific Inflammation: T-Cell Activation, CD4+ T (Th1/17) Cells, and CD8+ T (Tc1/Tc17) Cells
Following the antigen nonspecific inflammation, T-cell–mediated antigen-specific inflammation is initiated. When T cells infiltrate the skin, they are activated by cutaneous APCs, and produce cytokines such as IFN-γ and IL-17. In fact, stable interaction between skin DCs and T cells was observed in live imaging analysis [34] (Fig. 20.1b), and inhibition of CD86 expression by siRNA resulted in reduced inflammation [35], suggesting that effector T cells are activated locally by hapten-carrying APCs. Intriguingly, it has been reported that depletion of skin DCs in hapten-sensitized mice enhanced the effector phase of CHS [36], suggesting the existence of some DC subsets that play a regulatory function in the elicitation phase.
Cytokines produced by activated T cells then stimulate skin resident cells, which lead to further recruitment of T cells and amplify the inflammation. Each T-cell subset (i.e., Th1/Tc1 , Th2, Th17 /Tc17) activates the skin resident cells differently and forms their specific type of inflammation.
CHS was first considered to be a CD4+ T cell-mediated response as a representative of delayed-type hypersensitivity, but it is now recognized that both CD4+ and CD8+ T cells are important in the elicitation of CHS. CD8+ T cells mainly have pro-inflammatory effector functions, whereas CD4+ T cells have both pro- and anti-inflammatory functions that are dependent on their cytokine production pattern or subset.
CD4+ T helper (Th) cells and CD8+ T cytotoxic (Tc) cells can be subdivided into at least three subsets that are relevant for cutaneous immune responses: Th1/Tc1, Th2/Tc2, and Th17/Tc17 cells, respectively. Th1/Tc1 cells are characterized by the secretion of interferon (IFN)-γ; Th2/Tc2 cells by IL-4, IL-5, and IL-13; and Th17/Tc17 cells by IL-17A and IL-22 production. Although there exists some controversy regarding roles of each cytokine in CHS, the general trend is that IFN-γ from Tc1 is the major effector cytokine that provokes inflammation [37], and IFN-γ from Th1 and IL-17 from Th17/Tc17 also contribute for the full development of CHS [38–40]. It remains unknown whether Th2 cells contribute to the development of CHS, but several reports suggest that Th2 cells may also play important roles for the development of CHS in certain situations, depending on mouse strain or haptens [41].
IFN-γ or IL-17 produced by activated T cells induces various chemokine production from keratinocytes. Keratinocytes are an important source of chemokines in skin, and produce multiple chemokines, such as CXCL1, CXCL2, CXCL9, CXCL10, CCL8, CCL17, and CCL27. CXCL10 is a ligand for CXCR3, which are strongly expressed on Th1 cells and regulates their infiltration into skin [42, 43]. The CCL27-CCR10 and CCL17/22-CCR4 axes are another important mechanism for T-cell recruitment to skin [44, 45]. Neutrophil-recruiting chemokines also play important roles, because a blockade of CXCL1 or a deficiency of its receptor (CXCR2) leads to reduced CHS [32, 46].
20.5 Regulation of Inflammation: Regulatory T Cell (Treg)s
Evidence has accumulated regarding the regulatory mechanisms of Tregs in CHS [47]. Transfer of Tregs before elicitation suppresses the ear-swelling response [48], and depletion of endogenous Tregs before sensitization or elicitation enhances CHS response [49–51], indicating that Tregs play essential roles not only for the resolution of inflammation but also the initiation of T-cell priming. Studies of nickel allergy illustrated the existence of antigen-specific Tregs in healthy control individuals [52], suggesting the important roles of Tregs for the tolerance to allergens. As for the Tregs suppression mechanisms in CHS, several mechanisms such as IL-10 or CD39/73 dependent pathways are proposed [48, 53]. Table 20.1 summarizes the recent reports of Tregs function in CHS. Moreover, it has been revealed that Tregs display their inhibitory function by recirculating from skin to the draining [50]. As skin is an organ full of Tregs, such inhibitory mechanisms may also work in other skin diseases.
Table 20.1
An overview of recently published papers about Tregs and CHS
Major findings | References | |
|---|---|---|
Sensitization | Attenuated sensitization by Treg induced by orally administered antigen in an oral tolerance model | [54] |
Treg attenuate sensitization by modifying DC function through gap junction formation | [55] | |
Treg acquire an activated phenotype by means of ATP in draining LNs | [56] | |
Enhanced ear swelling response resulting from the depletion of endogenous Treg | [49] | |
Enhanced ear swelling response resulting from the depletion of endogenous Treg | [51] | |
Elicitation | Reduced ear swelling response resulting from the inhibition of the leukocyte influx through IL-10 from Treg | [48] |
Reduced ear swelling response resulting from the inhibition of the leukocyte influx through adenosine from Treg via CD39/CD73 (inhibition of E- and P-selectin expression in endothelial cells) | [53] | |
Treg acquire activated phenotype by means of ATP in blood | [56] | |
Enhanced and prolonged ear swelling response resulting from depletion of endogenous Treg | [50] | |
Prolonged ear swelling response resulting from depletion of endogenous Treg | [51] |
20.6 Conclusion
Taken together, the broad view of CHS development during the sensitization and elicitation phases is summarized in Fig. 20.2 and Table 20.2.
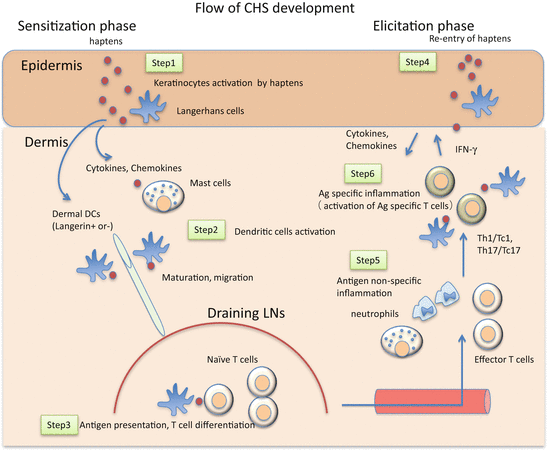

Fig. 20.2
A schematic view of the development of CHS. Step 1: Haptens activate keratinocytes (KCs) and mast cells directly or indirectly through innate immune systems. The activated KCs and mast cells produce various chemical mediators, which activate cutaneous DCs. Step 2: The activated DCs capture antigens and start maturation and migrate to the dLNs via afferent lymphatics. Step 3: Migrated DCs present antigen to naïve T cells in dLNs. Antigen-specific clones differentiate and proliferate into effector T cells. Tregs affect DCs function and play a suppressive role in effector T cell generation. Step 4: Upon re-exposure to haptens, KCs and mast cells are activated and produce various chemical mediators, which activate endothelial cells and cause inflammatory cell infiltration, including antigen-specific T cells. Step 5: Infiltrated antigen-specific effector T cells are activated and produce pro-inflammatory cytokines and chemokines, which activate KCs and cause further inflammatory cell infiltration. Step 6: In addition to effector T cells, Tregs infiltrate inflammatory sites and exert a suppressive function. Some infiltrated Tregs return to dLNs and may contribute to the resolution of inflammation
Table 20.2
Summary of the functions of immune cells in CHS
Cell subsets | Sensitization phase | Elicitation phase | |
|---|---|---|---|
Keratinocytes | Initiation of DC migration and maturation via production of TNF-α, IL-1β, IL-18, PGE2 | Recruitment of inflammatory cells through chemokine (CXCX1, CXCL2, CCL17, CCL27, CXCL9, CXCL10) production | |
Mast cells | Initiation of DC migration and maturation via TNF-α | Endothelial activation and promotion of inflammatory cell infiltration via TNF-α, histamine, CXCL1 | |
Neutrophils | Production of pro-inflammatory cytokines (TNF-α, etc), which contributes to Ag-nonspecific inflammation and subsequent Ag-specific inflammation | ||
Cutaneous dendritic cells | Langerhans cells | Ag presentation in dLNs to promote or suppress T cell differentiation and proliferation. Peak of migration: around 72–96 h after haptens application | Possible Ag presentation in dLNs and skin |
Langerin+dDCs (approximately 10 % of dDCs) | Ag presentation in dLNs to promote effector T cell differentiation and proliferation. Peak of migration: around 24–48 h after haptens application | ||
Langerin–dDCs (approximately 90 % of dDCs) | Ag presentation in dLNs to promote effector T cell differentiation and proliferation | ||
T cells | Th1/Tc1 | IFN-γ production: stimulate KCs to produce chemokines, and amplify the inflammation | |
Th2 | IL-4 production: stimulate/regulate the inflammation | ||
Th17/Tc17 | Promotion of effector T cell generation by IL-17 | IL-17 production: stimulate KCs to produce chemokines and amplify the inflammation | |
Treg | Suppression of T cells differentiation and proliferation by inhibiting the function of DCs | Suppression of endothelial cell activation and inhibition of T cell infiltration via IL-10 and/or adenosine degradation through CD39/73 |
The CHS model has provided us with valuable lessons on the mechanisms of ACD as discussed above. However, there still remains a compelling need to reveal whether such findings in CHS are relevant to human ACD. In addition, recent reports suggest that innate immune cells, such as innate lymphoid cells, macrophages, natural killer T cells, or gamma-delta T cells contribute to the development of many more diseases than previously thought. Investigation of the roles of such cells in CHS would also be important for the understanding of ACD, which may lead to an innovative therapy for allergic skin inflammation.
References
1.
Peiser M, Tralau T, Heidler J, Api AM, Arts JH, Basketter DA, English J, Diepgen TL, Fuhlbrigge RC, Gaspari AA, Johansen JD, Karlberg AT, Kimber I, Lepoittevin JP, Liebsch M, Maibach HI, Martin SF, Merk HF, Platzek T, Rustemeyer T, Schnuch A, Vandebriel RJ, White IR, Luch A (2012) Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell Mol Life Sci 69(5):763–781. doi:10.1007/s00018-011-0846-8 CrossRefPubMedPubMedCentral
2.
Honda T, Egawa G, Grabbe S, Kabashima K (2013) Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol 133(2):303–315. doi:10.1038/jid.2012.284 CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








