Fig. 15.1
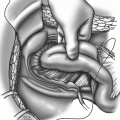
Port sites for the hybrid technique. During the initial laparoscopic phase, 5-mm ports are introduced via arm 1 (R 1 ), arm 2 (R 2 ), and arm 3 (R 3 ) for laparoscopic dissection. These are later replaced by 8-mm ports during docking for the robotic phase. C camera-lens system
2.
The laparoscopic phase: A 30° laparoscope is introduced via a subumbilical 12-mm port. Laparoscopic mobilization of the sigmoid colon is carried out through the two 5-mm ports at the right iliac fossa, and the assistant surgeon provides countertraction through the 5-mm port at the left iliac fossa. The sigmoid colon is mobilized, and the inferior mesenteric pedicle is controlled and divided using a standard technique [20]. Provided the intended level for anastomosis in the sigmoid can reach the true pelvis without tension, a complete splenic flexure mobilization is not always mandatory. Take down of the full splenic flexure is only indicated when there is inadequate length (colon or mesentery) or when the sigmoid is unhealthy (e.g., preoperative radiotherapy) or diseased (e.g., chronic diverticulitis). Even if take down of the splenic flexure is indicated, it is preferable to defer this until after full rectal mobilization because a fully mobilized left colon often falls down into the pelvis and hinders subsequent robotic pelvic dissection.
3.
Docking: After the left colon is adequately mobilized, the three 5-mm ports in the lower abdomen are replaced with 8-mm ports. The 30° laparoscope is removed, and docking is carried out. With experience, the docking time can be as short as 5 min. The robotic camera-lens system is finally inserted via the 12-mm subumbilical port (Fig. 15.1). The positions of the robot, monitors, and the operating team are shown in Fig. 15.2.
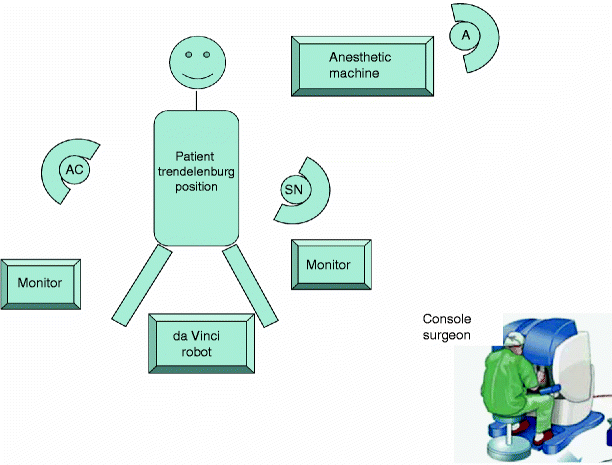

Fig. 15.2
Position of the operating team. Throughout the procedure, the patient is predominantly kept anesthetized in the Trendelenburg position with a right-side down-tilt. AC assistant surgeon, SN scrub nurse, A anesthetist
4.
The robotic phase: Using laparoscopic vision, the ProGrasp forceps are introduced via arm 3 (R3) and the permanent cautery hook and Maryland bipolar forceps are introduced via arm 1(R1) and arm 2 (R2), respectively (Fig. 15.1). The console surgeon holds up the rectum with the ProGrasp forceps and performs the dissection with arm 1 and arm 2. Additional retraction and suction/irrigation are provided by the assistant surgeon via two ports in the upper abdomen (Fig. 15.1). Posterior dissection is performed first and the plane between the mesorectum and the presacral fascia is followed closely near the mesorectal fascial envelope using a combination of sharp and gentle blunt dissection. Perfect hemostasis should be the goal at all times. The hypogastric nerves on the presacral fascia are identified and preserved. As dissection continues deeper, the plane of dissection turns anteriorly, and it is useful to rotate the 30° laparoscope 180° upward. The posterior dissection is followed by lateral dissection on either side, where the rectum and the mesorectal envelope are carefully dissected off the nervi erigentes. Anteriorly, the rectum is dissected off Denonvillier’s fascia, which covers the seminal vesicles in men or the vagina in women. The endpoints of pelvic dissection are the pelvic floor (posteriorly) and the seminal vesicles and the prostate in men or the vagina in women (anteriorly). The distal rectum should be clearly seen as a rectal tube penetrating the pelvic floor muscles. With experience, the console time can be limited to ≤1 h.
5.
Undocking: After adequate pelvic dissection, the system is undocked.
6.
After the robotic instruments are disengaged, the lower 8-mm port (R3) in the right iliac fossa is changed to a 5- to 12-mm versaport, through which an endostapler is introduced to transect the rectum (distal to the tumor) after peranal cytocidal washout.
7.
Exteriorization and resection of the specimen: A 4- to 6-cm Pfannestiel incision is made for specimen retrieval. If the tumor is small, the specimen also can be extracted via the intended ileostomy site in the right iliac fossa. A wound protector is used as a parietal protective drape during specimen retrieval.
8.
Intracorporeal anastomosis: Pneumoperitoneum is re-established. Intracorporeal anastomosis is performed with the circular stapler using a standardized laparoscopic view.
9.
A covering loop ileostomy is raised over the premarked stoma site.
10.
A drain is placed into the presacral space and anchored.
11.
All the ports with fascial defects greater than 5 mm are closed to prevent incisional hernia development.
Postoperative Management
The management of patients after robotic-assisted laparoscopic rectal surgery is essentially similar to that of the traditional operation. A nasogastric tube is not usually required; if one is placed during surgery, it should be removed toward the end of the operation. The urinary catheter is kept in place for 1–2 days. Pneumatic compression stockings are removed and replaced by elastic stockings. Patients can take liquids after the operation, and most advance to a regular diet by the second or third postoperative day. They are encouraged to ambulate the day after operation, and the stockings are removed once the patient is walking. A drain, if used, usually is removed on the second postoperative day if the output is not excessive. Techniques for stoma care are taught to patients with a covering ileostomy. After robotic-assisted rectal surgery, patients generally suffer from only mild to moderate pain, and the use of narcotics is minimal. The majority are able to be discharged between the fifth and seventh postoperative day. For patients with a covering stoma, a contrast study is arranged to ascertain the integrity of the anastomosis. Ileostomy closure can be arranged as early as 2 weeks after the initial operation.
Conclusion
There is no doubt that robotic rectal cancer surgery is highly feasible. Surgical robots may well represent the next major leap forward in minimally invasive surgery by virtue of their improved dexterity, vision, and ergonomic comfort. Colorectal surgeons without extensive laparoscopic experience who wish to acquire the sophisticated skills necessary for laparoscopic TME may benefit from this new technology. The major concern that remains is the oncological outcome. Data from the CLASSIC trial found a small (though not statistically significant) increase in positive radial resection margins in patients undergoing laparoscopic TME compared with open surgery (12 % vs. 6 %) [21]. At present, it is uncertain whether the oncological outcomes of robotic-assisted rectal cancer excision are comparable (or superior) to those of conventional laparoscopic surgery because long-term data are lacking [22, 23]. Besides this, the machine is heavy and bulky, and a more spacious operating room is preferred. A well-designed, user-friendly robotic endolaparoscopic suite together with effective team training is of paramount importance. In addition, the current generation of the Da Vinci robotic system does not provide tactile feedback, and although robotically performed operations to date have not been shown to be associated with higher rates of complications than standard laparoscopic equivalents, there is a real risk of inadvertent tissue injury because the surgeon must rely on visual cues to estimate the tension exerted on tissue. It is hoped that further advances in research and technology will bring us smaller, cheaper, faster, and safer robots with an improvement in tactile feedback. Currently, robotic-assisted surgery has proven feasible in right hemicolectomy [24, 25




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree