CHAPTER 11 Connective Tissue Diseases in Pregnancy
Introduction
The connective tissue diseases are multisystem disorders characterized by circulating nonorgan-specific autoantibodies. Many of them are more prevalent in women of child-bearing age, so are not uncommonly encountered in pregnancy. Pregnancy is associated with suppressed cell-mediated immunity. Thus connective tissue diseases in general may remit or improve during pregnancy. However, this is not universal; for example, systemic lupus erythematosus (SLE) may flare or present in pregnancy, with disastrous consequences.
Rheumatoid arthritis
Up to 75% of women with rheumatoid arthritis experience improvement of both joint and extra-articular features during pregnancy1, although fewer than 20% are in complete remission2. Improvement usually begins during the first trimester, and rheumatoid nodules may disappear. However, 90% of those who experience remission suffer postpartum exacerbations, and there is an increased incidence of rheumatoid arthritis onset in the postpartum period. Pregnancy does not cause reactivation of the symptoms of quiescent juvenile rheumatoid arthritis. The majority of patients with active disease will experience improvement or total remission in the second half of pregnancy and more than 50% of patients will flare postpartum3. Infants of women who also have anti-Ro antibodies are at risk of neonatal lupus (see below). The relative safety of drugs used to treat connective tissue disease in pregnancy4 is shown in Table 11.1.
Table 11.1 Drug Treatment of Connective Tissue Disease in Pregnancy4
| Drug | Relative Safety |
|---|---|
| Prednisone | Safe (especially in doses of 20 mg daily or less) |
| Sulfasalazine | Safe (folate supplementation is necessary before and throughout pregnancy) |
| Aspirin (low doses) | Safe (doses above 150 mg/day should be discontinued before 32 weeks’ gestation) |
| Nonsteroidal anti-inflammatory drugs | Relatively contraindicated (use steroids in preference and stop before 32 weeks if essential) |
| Heparin | Safe (although osteoporosis is a risk with prolonged high doses of unfractionated heparin) |
| Gold | Contraindicated |
| Azathioprine | Safe |
| Hydroxychloroquine | Safe |
| Penicillamine | Contraindicated |
| Methotrexate | Contraindicated |
| Cyclophosphamide | Contraindicated |
| Warfarin | Relatively contraindicated (avoid during weeks 6–12) |
| Fondaparinux | Contraindicated during pregnancy and breastfeeding |
| Leflunomide | Contraindicated during pregnancy and breastfeeding |
| Tacrolimus | Safe especially at low doses |
| Mycophenolate mofetil (MMF) | Contraindicated during pregnancy |
| Infliximab | Contraindicated in pregnancy and breastfeeding |
Systemic lupus erythematosus
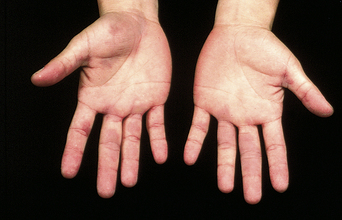
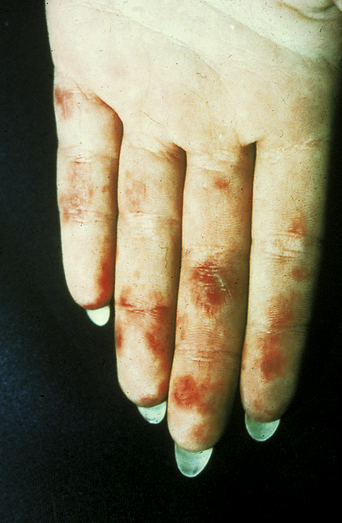
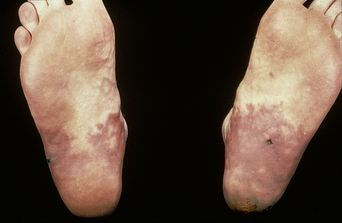
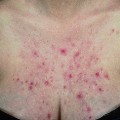
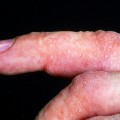
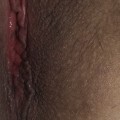
The prevalence of SLE is approximately 1 per 1000 women, but may be increasing. SLE flares may be difficult to diagnose during pregnancy because many features, such as hair fall, edema, facial erythema, fatigue, anemia, raised erythrocyte sedimentation rate, and musculoskeletal pain, also occur in normal pregnancy. About 60% of women with SLE have a flare during pregnancy or the puerperium, compared with about 40% of nonpregnant women over the same time period5. Cutaneous flares are the most common (Figures 11.1–11.3), followed by joint symptoms. Disease flares must be managed actively. Corticosteroids are the drug of choice4, but do not prevent flares. They should not be prescribed prophylactically, nor the dose increased for that purpose. Pregnancy does not seem to jeopardize renal function in the long term for women with lupus nephritis, although SLE nephropathy may manifest for the first time in pregnancy. There is a greater risk of deterioration in patients with a higher baseline serum creatinine level. A renal flare may be difficult to distinguish from pre-eclampsia, as hypertension, proteinuria, thrombocytopenia, and renal impairment are all features of both (Table 11.2).
Table 11.2 Features that Distinguish Renal Flare in Lupus Pregnancy from Pre-eclampsia
| Evidence of clinical lupus activity in other systems |
| Rising titer of anti-DNA antibodies |
| Evidence of alternate pathway of complement activation (i.e., ↓C3 or ↓C4) |
| Presence of cellular casts on urine microscopy |
| Absence of other features of pre-eclampsia (e.g., intrauterine growth retardation, abnormal liver function test results, hyperuricemia) |
SLE is associated with an increased risk of spontaneous abortion, fetal death, pre-eclampsia, preterm delivery (60%), and intrauterine growth restriction (IUGR)6. These adverse outcomes are associated with the presence of anticardiolipin antibodies (aCLs) or lupus anticoagulant (LA) (antiphospholipid antibodies; aPLs), lupus nephritis or hypertension, and either active disease at the time of conception or first presentation of SLE during pregnancy. In the absence of these features, the risk of adverse outcome is not increased.
Neonatal lupus syndromes
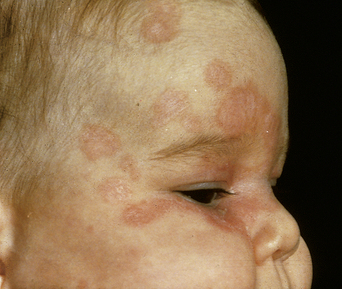
The commonest manifestation (5% risk if anti-Ro-positive) is cutaneous neonatal lupus. The eruption (Figure 11.4) usually appears in the first 2 weeks of life. The typical lesions tend to be geographical, annular, erythematous, and scaly, and occur over the face, scalp, or other light-exposed skin. The rash disappears spontaneously within 6 months and scarring is unusual.
The risk of congenital heart block is less (2–3% risk if anti-Ro-positive), and is usually detected in utero at around 18–20 weeks. The perinatal mortality rate is increased, with 20% of affected children dying in the early neonatal period. Most infants who survive this period do well, although 50–60% require a pacemaker. The risk of a second child being born with heart block is 25%, rising to 50% after two or more affected babies7.