(1)
Obesity Institute, Geisinger Medical Center, Southold, NY, USA
Abstract
The initial medical evaluation, which is conducted by a medical provider with familiarity with all obesity-related comorbid conditions that contribute to surgical risk, establishes the focus for the comprehensive medical evaluation. Extreme obesity is commonly associated with significant abnormalities in critical organ function, which are usually related to the extent and duration of obesity. The degree of obesity-related organ dysfunction must be carefully evaluated in order to complete a patient-centered risk assessment. Patients with significant cardio-pulmonary impairment should be evaluated for obese cardiomyopathy, pulmonary hypertension, obesity hypoventilation, severe sleep apnea, and thromboembolism risk. The comprehensive medical evaluation includes those specialty consultations and medical tests necessary to establish patient-centered health risks of extreme obesity and risks of bariatric surgery.
Extreme obesity is associated with many comorbid medical conditions that contribute to the risks of cardiovascular disability and death. Many patients who are interested in surgical weight loss will present with comorbid conditions that are undiagnosed, improperly evaluated, or inadequately treated. Bariatric Surgeons and their program personnel should have a solid understanding of the obesity disease burden, current recommendations for diagnosis and evaluation, prognosis, and implications regarding surgical risk. This understanding is essential for risk management, patient selection, and communication with consulting medical specialists. In general, the obesity disease burden, the number of comorbid conditions and their severity are influenced by increasing body mass index (BMI), increasing age, and male gender [1]. The duration of extreme obesity is another important factor, which influences the severity of the obesity disease burden.
A recent advisory from the American Heart Association addressing the evaluation and management of severely obese patients undergoing surgery calls attention to a number of clinical findings, which mandate additional testing (Table 6.1) [2]. Younger patients, especially women with BMI ≤45 kg/m2, who are free of these conditions and have a normal capacity for exercise will generally need little additional preoperative medical evaluation or testing.
Table 6.1
Clinical conditions associated with extreme obesity, which should influence preoperative cardiac assessment and management [2]
• Atherosclerotic cardiovascular disease |
• Congestive heart failure |
• Systemic hypertension |
• Pulmonary hypertension |
• Cardiac arrhythmia |
• Thromboembolism |
• Limited exercise capacity |
Respiratory Assessment
Respiratory conditions, respiratory symptoms, and limited exercise tolerance are commonly encountered in patients with extreme obesity. Even in the absence of demonstrable respiratory abnormalities, sedentary individuals with extreme obesity will have breathlessness with exertion that is related to deconditioning. Similarly, many inactive patients with extreme obesity may have significant impairment of respiratory function in the absence of symptoms.
Because of expanded adipose tissue and lean body mass, extreme obesity is associated with an increased oxygen consumption and carbon dioxide production with an increase in resting minute ventilation [3]. Additional physiologic alterations in pulmonary function associated with obesity are summarized in Table 6.2.
Table 6.2
Alterations in pulmonary function associated with severe obesity
• Reduced compliance of lungs and chest wall |
• Increased respiratory resistance |
• Increased work of breathing |
• Reduced lung volumes |
Increases in upper body fat, increased pulmonary blood volume, and closure of dependent airways can increase respiratory “stiffness” (reduce system compliance) to a significant degree [3]. These alterations, which increase the work of breathing, are associated with increasing BMI. The increase in respiratory work may be clinically important after upper abdominal surgery when additional temporary reductions in pulmonary function related to surgery and anesthesia increase the risk of respiratory failure.
Reduction in lung volume is the most frequently found respiratory alteration in severe obesity. Mild reductions in Total Lung Capacity (TLC), Vital Capacity (VC), and 1 min Forced Expiratory Volume (FEV1) are often seen, but the most profound reduction is in the Expiratory Reserve Volume (ERV), which is exponentially related to BMI (Fig. 6.1) [4]. The reduction in ERV occurs because the obese abdomen displaces the diaphragm into the thorax. ERV can reduce to as low as 20 % of predicted value [4], but the other component of the Functional Residual Capacity (FRC), the Residual Volume (RV), remains unchanged in extreme obesity. Significant reductions in FRC will predispose to small airway closure during normal tidal breathing, which results in ventilation–perfusion mismatch and hypoxia. These physiologic changes are most pronounced in the recumbent position, where maximal diaphragmatic displacement into the thorax occurs. Bariatric surgery patients with reduced FRC are at increased risk for dangerous hypoxia during endotracheal intubation after the pharmacologic induction of apnea, because the reduced FRC limits the safe duration of apnea. The reductions in FRC are correlated with increasing age, increasing BMI, and male gender [5].
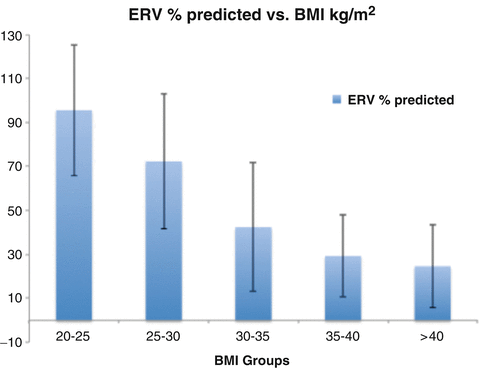

Fig. 6.1
The exponential relationship between increasing BMI (kg/m2) and the % predicted reduction in Expiratory Reserve Volume (ERV). BMI body mass index
An important component of the respiratory evaluation of bariatric surgery candidates is the Arterial Blood Gas (ABG) analysis. This valuable test is often overlooked in the preoperative assessment. Several studies have documented a significant prevalence of hypoxia and hypercarbia in these patients [6, 7]. ABG analysis is essential for accurate patient-centered risk analysis as it will enable diagnosis of the Obesity Hypoventilation Syndrome (OHS), a condition associated with a high mortality, and will identify patients at risk for postoperative respiratory failure. In addition, information from ABGs will facilitate the optimal management of anesthetic and perioperative respiratory care [6, 8]. Postoperative hypoxemia is common in bariatric surgery patients and correlates with reduced perioperative tissue oxygenation, which has been recently documented following bariatric surgery [9]. Tissue hypoxia will reduce tissue resistance to infection and interfere with wound healing.
A full pulmonary evaluation including chest film, pulmonary function tests (PFT), and ABG analysis is indicated for those bariatric surgery candidates who have documented pulmonary conditions, those with limited exercise tolerance because of dyspnea, those with a history of heavy smoking, and those with BMI ≥60 kg/m2.
Obstructive Sleep Apnea
Obstructive Sleep Apnea/Hypopnea Syndrome (OSAHS) is a recently discovered respiratory disorder that is a cause of daytime sleepiness, and disturbed sleep. It can cause mortality and contributes to surgical morbidity. The syndrome is caused by recurrent upper airways obstructions during sleep that cause hypoxia, increased respiratory effort, and frequent arousals. An apnea is a breathing pause lasting ≥10 s and a hypopnea is present during continuous breathing, when ventilation is reduced by at least 50 % for ≥10 s [10]. The Apnea–hypopnea Index (AHI) is the sum of apneas and hypopneas per hour of sleep. A diagnosis is established when the AHI is ≥5/h. During sleep, the negative pressure of inspiration closes the airway as the striated airway-dilating muscles relax. Muscle tone is reduced during sleep and the airway narrows causing snoring, apneas and hypopneas. This results in hypoxia, and then arousal from sleep. The arousal may be accompanied by cardiac acceleration, blood pressure rise, and increased sympathetic activity [10].
Surveys of the general population reveal that OSAHS is present in 9 % of adult women and 24 % of men [11]. This condition is much more common in obesity because subcutaneous fat and periluminal fat contribute to airway narrowing. In addition, reduced respiratory compliance further predisposes to airway closure. The actual prevalence of this syndrome increases as BMI increases [10]. In candidates for bariatric surgery, this condition is extremely common. A recent study from a bariatric center of excellence revealed an overall prevalence of 77 % in prospective candidates with 30.7 % having mild OSA (5 ≤ AHI ≤ 15), 19.3 % having moderate obstructive sleep apnea (OSA) (15 < AHI ≤ 30), and 27.2 % having severe OSA (AHI > 30) [12].
OSAHS is associated with hypertension, sudden cardiac death, heart failure, arrhythmias, and other conditions with cardiovascular risk [11]. In addition, preliminary recent evidence suggests a possible link with insulin resistance and steatohepatitis in severe obesity [13]. The syndrome is associated with perioperative respiratory complications because general anesthetics decrease upper airway dilator muscle activity, anesthetic medications will interfere with the arousal response, and narcotics will suppress respiratory drive [14]. The repetitive chronic airway collapse and hypoxia associated with this condition causes pulmonary arterial vasoconstriction, which will contribute to chronic vascular remodeling and pulmonary hypertension (PH) [15], a recently discovered risk factor in bariatric surgery [16].
Clues to the identification of this condition include the presence of polycythemia, a history of regular snoring, nocturnal gasping, choking, witnessed apneas, or daytime sleepiness (Table 6.3).
Table 6.3
Clinical clues to the identification of obstructive sleep apnea
• Polycythemia |
• Regular heavy snoring |
• Nocturnal gasping, choking |
• Witnessed apneas |
• Daytime sleepiness |
Several survey tools are available [17, 18] which may help facilitate the diagnosis. However, because of poor negative predictive values with these tools [12], many routinely refer bariatric surgical candidates for full polysomnography with the recording of multiple respiratory and neurophysiologic signals during sleep.
Candidates for bariatric surgery should have this condition evaluated and diagnosed early by polysomnography in order to allow time for preoperative treatment with Continuous Positive Airway Pressure (CPAP) during sleep. This treatment has been shown to improve sleep symptoms, cognition, mood, blood pressure, and hypoxia as well as reduce pulmonary artery pressures [19]. Unfortunately, the delays associated with managed care, precertification, and approval have interfered with the use of this treatment modality prior to bariatric surgery in patients who might benefit from this treatment.
Obesity Hypoventilation Syndrome (The Pickwickian Syndrome)
The association between daytime somnolence and obesity has been known for many years. The most famous early report of this condition consists of a case report by Burwell describing an obese individual who was hospitalized after falling asleep at a poker game holding a full house of aces and kings [20]. The syndrome is defined as daytime hypercapnea (PaCO2 ≥ 45 mmHg) and sleep disordered breathing (most commonly severe sleep apnea) and hypoxia (PaO2 < 70 mmHg) [21]. Other conditions that may be present at diagnosis include cor pulmonale, PH, hypersomnia with no other explanation, and erythrocytosis [22].
This condition is fairly common among candidates for bariatric surgery and may be missed in centers that do not check ABGs. The prevalence among patients with OSA is 20 % [23] and in a small study of 229 bariatric surgery candidates, 16 % had PaCO2 ≥ 45 [6]. The prevalence of OHS increases as BMI and AHI increase. The perception that this condition may go unrecognized in bariatric centers is supported by a study of 4,332 patients admitted to an internal medicine service, where 32 % met the diagnostic criteria for obesity hypoventilation. Of these patients, only a small fraction was given the diagnosis and received treatment. Short-term follow up of these patients demonstrated a high mortality in comparison to non-OHS patients with similar extent of obesity [24].
OHS patients are usually discovered in their 50s or 60s. They are usually morbidly obese (BMI ≥40 kg/m2), and have OSA with an AHI in the severe range [21]. Most have classic symptoms of OSA, which include loud snoring, nocturnal choking, witnessed apneas, excessive daytime somnolence and morning headaches. Physical findings often include increased neck circumference, oropharyngeal crowding, a loud pulmonary second sound (may be impossible to identify in extreme obesity), and lower extremity edema [22, 23, 25]. Although the definitive test for the diagnosis is an ABG performed on room air, additional findings, which suggest OHS include an abnormal oxygen saturation detected on finger oximetry [21, 22] and an elevated bicarbonate level. Chau et al. developed a handy decision tree which demonstrates that OHS is unusual if the serum bicarbonate level is <27 meq/l, and that a bicarbonate level ≥27 meq/l, coupled with an AHI ≥100 has a strong association with OHS [26]. Major physiological differences between patients with OHS and OSA with eucapnia include an increase in respiratory load, an impaired CNS response to hypoxia and hypoventilation, and impaired neurohumoral responses (leptin resistance) [25].
OHS must be considered among candidates for bariatric surgery because it is a proven risk factor for mortality and perioperative adverse events. In addition, OHS is commonly associated with PH and right ventricular dysfunction or failure. PH is now an established risk factor for postoperative adverse events and mortality following bariatric surgery [16]. If the diagnosis of OHS is suspected, pulmonary consultation is indicated to rule out other causes of hypoventilation and to initiate treatment. In addition, cardiac ultrasound is indicated to look for PH and right ventricular dysfunction. If PH is found, more definitive studies are indicated (please refer to the discussion of “Pulmonary Hypertension” on this chapter).
If OHS is present and bariatric surgery is contemplated, treatment of OHS with CPAP or bi-level ventilation is indicated. This treatment will improve gas exchange and pulmonary hemodynamics, which will improve working conditions for the right ventricle. Short-term positive airway pressure for ≤3 weeks will reduce nocturnal hypoxia, improve hypercarbia and improve breathing during sleep. Longer-term therapy will improve lung volumes, improve ventilator response to CO2, and reduce mortality [26]. CPAP treatment should be supervised by a Pulmonary Medicine specialist who ideally should document the improvement with treatment and share the details of this with the bariatric team. In addition, for severe cases, tracheostomy should be considered before bariatric surgery, as this will improve gas exchange and facilitate perioperative positive pressure ventilation.
Cardiac Assessment
Obesity is a well-known risk factor for atherosclerosis and cardiovascular disease. Comorbid conditions in association with obesity including hypertension, insulin resistance, and hyperlipidemia are commonly referred to as the “metabolic syndrome” and have a strong association with cardiovascular morbidity and death. The long-standing association between obesity and heart failure is related to the presence of the metabolic syndrome with an associated chronic inflammatory state, abnormal endothelial function, and hypercoagulability [27]. The profound favorable changes in cardiovascular health that occur in association with weight loss success after bariatric and metabolic surgery are a major driving force behind the rapid acceptance of bariatric surgery as the treatment of choice for extreme obesity [28].
In conjunction with the introduction and evolution of bariatric surgery, cardiologists have studied the structure and function of the heart in patients with extreme obesity and have discovered consistent patterns of alterations in cardiac structure and function as well as profound hemodynamic changes. The expanded adipose tissue mass and supporting lean body mass in extreme obesity will increase total body oxygen requirements which necessitates compensatory increases in circulatory and respiratory demands. Adipose tissue, which is now known to be highly metabolically active, commands substantial blood flow (7.4 ml blood flow/100 g adipose tissue/min) [29]. This has been translated to a blood flow of 3 l/min for 100 kg of fat [30]. In extreme obesity, blood volume is expanded resulting in increased preload, which supports the increase in cardiac output [30]. Autopsy studies of patients with extreme obesity show evidence of enlarged cardiac chambers and cardiac muscular hypertrophy with the extent related to the degree of obesity [31]. Studies in echocardiography have been performed in large numbers of obese patients, compared with lean controls, and demonstrate the combination of dilatation of cardiac chambers and hypertrophy of ventricular muscle, with incidence related to extent and duration of obesity [32].
Studies in morbidly obese patients with and without congestive heart failure (CHF) indicate that heart failure in obesity is accompanied by increases in cardiac output, right sided filling pressures, pulmonary artery pressures, and pulmonary capillary wedge pressures [30, 32]. In addition, those morbidly obese patients with heart failure had larger chamber size and more ventricular hypertrophy than those without heart failure [32]. The presence of heart failure is correlated via sigma curve with the duration of extreme obesity [32]. In patients with associated OSA or OHV, hypoxic vasoconstriction and vascular remodeling will cause increased pulmonary artery pressures with a gradient between pulmonary capillary wedge pressure and pulmonary artery diastolic pressure [30].
The problem of pulmonary congestion in extreme obesity is frequently complicated by abnormalities in diastolic function that are frequent in extreme obesity. The hypertrophied left ventricle in extreme obesity is stiffer with reduced compliance resulting in impaired left ventricular filling in diastole. As with left ventricular mass, the duration of obesity influences the extent of diastolic dysfunction [30]. Many bariatric centers have evaluated patients with long-standing extreme obesity, who have a past history of CHF treatment, and yet, left ventricular systolic function and ejection fraction are normal. These patients have what is now termed diastolic heart failure [33]. The studies of systolic function in extreme obesity yield conflicting results. A study of 43 morbidly obese patients with heart failure revealed a mean cardiac output 5.62 ± 1.48 l/min with a cardiac index of 2.3 ± 0.55 l/min/m2 [32]. Noninvasive studies of systolic function demonstrate both preserved and depressed systolic function in association with heart failure in severe obesity [30].
The increased circulatory demands in extreme obesity result in an increase in cardiac output, stroke volume and stroke work. In order to facilitate the increased blood flow requirements, systemic vascular resistance falls. The increased cardiac output leads to chamber dilatation, which increases wall stress. In order to compensate for the increase in wall stress and to preserve systolic function, eccentric ventricular hypertrophy occurs. If ventricular hypertrophy and muscle function can keep pace with the increases in wall stress, systolic function is maintained. If hypertrophy cannot adequately compensate for wall stress, systolic function will decrease. In these patients, the heart failure will involve systolic and diastolic ventricular failure. Autopsy studies have demonstrated areas of cardiac muscle necrosis and fibrosis and areas of myocardial fatty deposition (steatosis) in the hypertrophied left ventricle. There is recent interest in the concept of fatty degeneration of the heart as a contributor to obese cardiomyopathy [34]. The interested reader is referred to an excellent review of the pathogenesis of the cardiomyopathy of obesity [30]. The changes in cardiovascular structure and function associated with extreme obesity are summarized in Table 6.4.
Table 6.4
Cardiovascular physiologic alterations which are associated with extreme obesity
• Increased resting heart rate |
• Increased resting cardiac output |
• Increased resting stroke volume |
• Increased ventricular wall thickness |
• Decreased maximal exercise O2 consumption |
The cardiomyopathy of obesity is present in 31 % of patients with morbid obesity, especially those with long standing extreme obesity [30]. Clues to the diagnosis include a recent weight gain accompanied or followed by dyspnea with exertion, paroxysmal nocturnal dyspnea, orthopnea, or extremity edema. At this time, if investigated, systolic function is usually normal. Atrial fibrillation or atrial flutter may be present. When cardiomyopathy occurs with OSA or OHV, signs of right heart failure will be present. Physical findings including gallop (S3, S4 heart sounds) rhythm, pulmonary rales, jugular venous distension, hepatojugular reflux, and extremity edema. Many of these physical findings will be missed due to the thickness of subcutaneous fat.
Atherosclerotic cardiovascular disease is also common in extreme obesity, especially in association with metabolic syndrome. Coronary artery disease, if occult or undiagnosed, will increase the risks of bariatric surgery. The prevalence of coronary artery disease in extreme obesity is unknown, but cardiac complications following bariatric surgery occur in 0.7–1.5 % [35]. The Revised Cardiac Risk Index [36] has been useful for the identification risk factors for perioperative cardiovascular complications in the general population (Table 6.5).




Table 6.5
Risk factors for perioperative cardiovascular complications in the general population from the Revised Cardiac Risk Index [36]
• Major surgery (abdominal, thoracic, vascular) |
• Coronary artery disease (myocardial infarction, chest pain, previous coronary revascularization) |
• Congestive heart failure |
• Cerebrovascular disease |
• Preoperative treatment with insulin |
• Preoperative creatinine levels >2 mg/dl |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








