27 Complications of permanent fillers
Summary and Key Features
• Permanent fillers comprise mostly synthetic materials that cause collagen deposition via fibroplasia as their mechanism of action
• Permanent fillers are better at facial volumizing and deep structural augmentation than at ‘line filling’
• Silicones, polyalkylimides, polyacrylamides, polymethylmethacrylate, and acrylic hydrogels are the most common permanent fillers worldwide
• All cosmetic fillers, whether temporary or permanent, may induce adverse reactions
• Permanent filler complications may be due to the injection itself, injector-dependent variables, or host tissue and filler interactions
• Permanent filler complications are difficult to treat since the product will not dissipate, but rather remains in vivo
• Foreign-body granulomas and late-onset granulomas are the most challenging permanent filler complications to treat
• Late-onset granulomas are sometimes due to biofilms from bacteria introduced at the time of injection
• Effective treatments of granulomas must target bacterial etiologies and host immune response mechanisms
• Invasive and scarring treatments such as surgery should be reserved and used after more conservative treatments have failed
Products (Box 27.1, Table 27.1)
Table 27.1 Selected permanent fillers by category and composition
| Category | Branded product | Composition |
|---|---|---|
| Liquid injectable silicone | Silikon-1000® | Purified polydimethylsiloxane polymer |
| Adatosil-5000® | Purified polydimethylsiloxane polymer | |
| Other ‘silicones’ | Adulterated and unknown products | Variable and often unknown |
| Polyalkylimide gels (hydrophilic) | Bio-Alcamid® | 3% or 4% polyalkylimide gel in 97% or 96% sterile water |
| Polyacrylamide gels (hydrophilic) | Amazingel® | Polyacrylamide gel in sterile water |
| Aquamid® | 2.5% polyacrylamide gel in 97.5% sterile water | |
| Polymethylmethacrylate (hydrophobic) | Arteplast® (first generation) | 30–42 µm PMMA microspheres in a gelatin carrier |
| Artecoll® (second generation) | 30–50 µm PMMA microspheres in a bovine collagen carrier | |
| Artefill® (third generation) | 30–50 µm PMMA microspheres in a bovine collagen carrier | |
| Metacril® | PMMA in carboxyglutamate | |
| Acrylic hydrogel (hydroxyethyl-methacrylate / ethylmethacrylate) (hydrophobic) | Dermalive® | 45–65 µm polygonal fragments acrylic hydrogel (40%) in HA (60%) |
| Dermadeep® | 80–110 µm polygonal fragments acrylic hydrogel (40%) in HA (60%) |
HA, hyaluronic acid; PMMA, polymethylmetacrylate.
Polyalkylimide gel
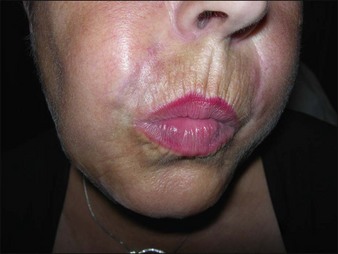
Bio-Alcamid® (Polymekon, Italy) was first launched in 2000 as an ‘endoprosthesis’ for the treatment of pectus excavatum, postoperative defects, and aesthetic use in the lips, cheeks, and nasolabial folds (Fig. 27.1). It contains a non-degradable, atoxic, non-immunogenic synthetic polyalkylimide cross-linked polymer suspended in water. Although it is approved in Europe, it is not currently approved by the FDA. Bio-Alcamid® contains a ratio of either 3% or 4% alkylimide polymer to 97% or 96% sterile water. The product is not a particulate one, but rather a gel that is injected as a large bolus. It is meant to be injected subcutaneously rather than into the dermis. It does not require allergy testing and has been histologically shown to induce fibroplasia once implanted.
Polyacrylamide gels
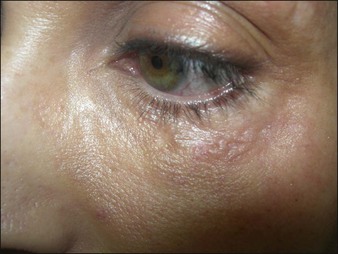
Aquamid® (Contura International, Denmark) contains a 2.5% cross-linked polyacrylamide gel in 97.5% sterile water. It has been in use in Europe and worldwide since 2001, primarily for augmentation of nasolabial folds and lips, facial contouring, and for correction of HIV facial lipoatrophy (Fig. 27. 2). It is also a viscoelastic gel without particles. On histology, Aquamid® appears as a multivacuolated non-birefringent material with a surrounding layer of thin fibrous connective tissue, macrophages, multinucleated giant cells, and lymphocytes.
Polymethylmethacrylate
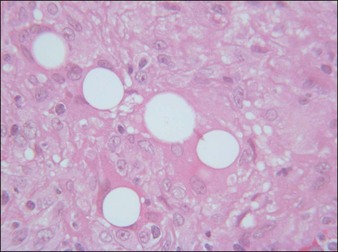
Artecoll® (Rofil Medical International, Breda, The Netherlands) (Fig. 27.3) was then introduced in 1992 as a second-generation product with an improved processing and washing method that reduced impurities. Significantly, the carrier was also reformulated, and the PMMA was suspended as a 20% product in 80% bovine collagen with 0.3% lidocaine for improved comfort. Because of the bovine collagen component, Artecoll® requires allergy testing prior to injection. As such Artecoll® is considered a biphasic implant, inducing augmentation through displacement with an early collagen component and later through a fibroplastic component caused by PMMA. In 1998, Artecoll® was approved in Canada, and processing methods were further improved to reduce particle size variability, resulting in fewer incidences of late granuloma formation compared with Arteplast®. Histology of skin biopsy specimens from areas treated with Artecoll® show round, sharply circumscribed, translucent, non-birefringent particles, epithelioid histiocytes, multinucleated giant cells, lymphocytes, and occasional eosinophils surrounding the microspheres.
Acrylic hydrogel plus hyaluronic acid
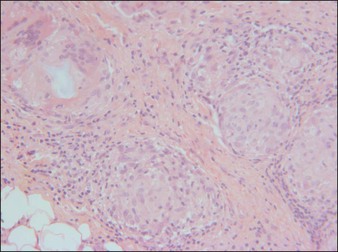
Dermalive® and Dermadeep® (Dermatech, Paris, France) are biphasic permanent fillers composed of a mixture of 40% hydroxyethylmethacrylate and ethylmethacrylate particles and 60% biodegradable, fluid cross-linked, bacterially derived HA (Fig. 27.4). The acrylic particles are hydrophobic and irregularly shaped in a polygonal fashion. Dermalive® contains acrylic particles 45–65 µm in diameter, whereas Dermadeep® contains particles 80–110 µm in diameter. Both products are intended for injection in the deep dermis and subcutaneous regions, with the Dermadeep product indicated for deep subcutaneous injection only. As with most permanent fillers, injections into the superficial and mid-reticular dermis are not recommended. Both products were CE approved and available in Europe in 1998, but were withdrawn several years ago owing to the high incidence of complications. Neither product has ever been FDA approved for use in the USA. Histology of acrylic hydrogels in tissue reveals pseudocystic structures of different sizes and shapes containing polygonal, pink, translucent, non-birefringent foreign bodies with a surrounding granulomatous reaction of epithelioid histiocytes, multinucleated giant cells, some lymphocytes, and occasional eosinophils surround the microstructures.