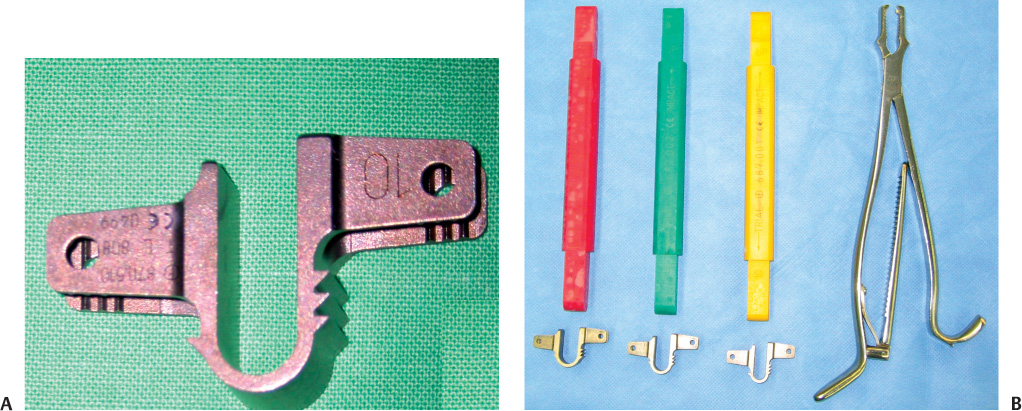
34 Spinal Stenosis with or without Segmental Instability Adjacent Level to Rigid Fixation Elderly Patients and Osteoporotic Patients with Segmental Instability Since neurogenic claudication secondary to spinal stenosis was first described by Verbiest,1 decompressive surgery has been required in patients who failed to respond to conservative therapy. However, decompressive surgery sometimes leads to secondary instability. In cases where secondary instability is expected to develop following decompression of the posterior column as well as in cases with instability, additional posterior fusion has been indicated. Although its importance in surgery for spinal stenosis cannot be neglected, posterior fusion is not an ideal treatment modality. Studies have shown that this procedure can result in the adjacent segmental syndrome.2–4 If surgeons perform fusion surgery with a concern for postoperative potential instability, too many fusions may be done unnecessarily. In addition, rigid fixation may fail to fuse in elderly, osteoporotic patients. Interspinous implants have been invented to treat lumbar neurogenic claudication secondary to spinal stenosis.5,6 The indication of Coflex—interspinous implantation—includes minor segmental instability in patients requiring surgery for either or both disk herniation and central spinal canal stenosis. It is implanted in the interspinous space, originally after removal of the interspinous ligaments and resection of their bony attachment. By preventing extension, it relieves the symptoms of lumbar spinal stenosis. However, it cannot be used as a substitute for a rigid fusion in cases of marked instability.5 French orthopedic surgeon, Jacques Samani, invented the Interspinous “U” in 1994.7 The Interspinous “U” was renamed the Coflex, which is designed to be inserted between two adjacent lumbar spinous processes (Fig. 34–1). Coflex Interspinous Implant (Paradigm Spine, LLC, New York, NY) is a titanium device with a U-shaped body and two wings on each side. The device provides flexible support of the posterior column. Coflex is designed to permit flexion of the lumbar spine, although it restricts mobility in extension and rotation. Coflex can be applied from L1 to L5 (sometimes S1) because it needs the support of the spinous process. Coflex is available in five heights 8, 10, 12, 14, and 16 mm (Fig. 34–1B). The main controversy about the interspinous implant is its clinical efficacy. Because it is designed to prevent hyperextension, theoretically it can be utilized in any case in which extension aggravates the neurogenic pain. Degenerative spinal stenosis can be an ideal indication, whereas simple disk herniation or spinal stenosis with severe instability is not an indication of this implant. Overuse of this implant may lead to increased medical cost and prolonged operation time. Until now, the indications for restabilization by interspinous implant documented by many reports are as follows: 1. Spinal stenosis with or without segmental instability 2. Intraoperative instability 3. Adjacent level to rigid fixation 4. Elderly patients or osteoporotic patients with segmental instability We define the mild segmental instability on the standing x-ray lateral film as (1) degenerative spondylolisthesis, grade I (<4 mm in the sagittal plane), or (2) angular instability [intervertebral range of motion (ROM) > 10 degrees or intervertebral angle (flexion) <0 degrees], or (3) mild retrolisthesis. In addition, this includes conditions that aggravate low back pain and develop referred pain by motion, even if there is no radiological instability. Figure 34–1 (A) The sagittal view of Coflex. This device is designed to be placed between two adjacent spinous processes. Implant migration is prevented by the clamping of the lateral wings. (B) Sizes of Coflex and its template. Although preoperative evaluation reveals no instability clinically and radiologically, one may encounter segmental instability during surgery. This can be assessed objectively by placing active traction on the spinous processes utilizing clamps during surgery; a significant degree of motion induced by mild traction means decreased stability.5 Coflex is designed as a cushioning mechanism adjacent to fusion as well as a stand-alone distractor for spinal instability.7 It is not accurately known whether Coflex adjacent to long, rigid fusion may be effective or not, but this can be used as an adjunctive therapy for stabilizing levels above or below a fusion (topping off) to minimize adjacent level degeneration. Elderly and osteoporotic patients show lower fusion rates by rigid fixation. These patients tend to have more chances of complication such as loosening of screw fixation and failure of fusion. Therefore, Coflex can be an alternative of rigid fixation in these patients. Meanwhile, contraindications of Coflex include severe segmental instability, progressive degenerative spondylolis-thesis (grade 2 or higher), kyphosis, severe scoliosis, and isthmic spondylolisthesis. Although Coflex was applied previously in some instances, complications such as slippage of the device and progression of spondylolisthesis occurred.
Coflex
Product Design
Indications/Contraindications
Spinal Stenosis with or without Segmental Instability
Intraoperative Instability
Adjacent Level to Rigid Fixation
Elderly Patients and Osteoporotic Patients with Segmental Instability
Operative Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Product Design
Product Design Indications/Contraindications
Indications/Contraindications Operative Technique
Operative Technique Preliminary Results
Preliminary Results Radiological Outcome
Radiological Outcome Complications
Complications Discussion
Discussion Conclusion
Conclusion